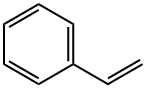
Styrene , StandardforGC,>99.5%(GC) , 100-42-5
Synonym(s):
Phenylethylene;Phenylethylene, Vinylbenzene;Styrene;Vinylbenzene
CAS NO.:100-42-5
Empirical Formula: C8H8
Molecular Weight: 104.15
MDL number: MFCD00008612
EINECS: 202-851-5
PRODUCT Properties
| Melting point: | -31 °C (lit.) |
| Boiling point: | 145-146 °C (lit.) |
| Density | 0.906 g/mL at 25 °C |
| vapor density | 3.6 (vs air) |
| vapor pressure | 12.4 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | 88 °F |
| storage temp. | Store at <= 20°C. |
| solubility | 0.24g/l |
| form | Liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| Specific Gravity | 0.909 |
| color | Colorless |
| Odor | at 0.10 % in triacetin. sweet balsam floral plastic |
| Odor Type | balsamic |
| biological source | synthetic |
| Odor Threshold | 0.035ppm |
| explosive limit | 1.1-8.9%(V) |
| Water Solubility | 0.3 g/L (20 ºC) |
| FreezingPoint | -30.6℃ |
| Sensitive | Air Sensitive |
| Merck | 14,8860 |
| BRN | 1071236 |
| Henry's Law Constant | (x 10-3 atm?m3/mol):
3.91 at 25 °C (static headspace-GC, Welke et al., 1998) |
| Exposure limits | TLV-TWA 50 ppm (~212 mg/m3) (ACGIH
and NIOSH), 100 ppm (~425 mg/m3)
(OSHA and MSHA); ceiling 200 ppm, peak
600 ppm/5 min/3 h (OSHA); STEL 100 ppm
(~425 mg/m3) (ACGIH). |
| Dielectric constant | 2.4(25℃) |
| Stability: | Stable, but may polymerize upon exposure to light. Normally shipped with a dissolved inhibitor. Substances to be avoided include strong acids, aluminium chloride, strong oxidizing agents, copper, copper alloys, metallic salts, polymerization catalysts and accelerators. Flammable - vapour may travel considerable distance to ignition source |
| InChIKey | PPBRXRYQALVLMV-UHFFFAOYSA-N |
| LogP | 2.96 at 25℃ |
| CAS DataBase Reference | 100-42-5(CAS DataBase Reference) |
| IARC | 2A (Vol. 60, 82, 121) 2019 |
| NIST Chemistry Reference | Styrene(100-42-5) |
| EPA Substance Registry System | Styrene (100-42-5) |
Description and Uses
Styrene has a characteristic, sweet, balsamic, almost floral odor that is extremely penetrating. Styrene occurs naturally in plants. It was first isolated from a resin called storax obtained from the inner bark of the Oriental sweet gum tree (Liquidambar orientalis) by Bonastre. In 1839, the German apothecary Eduard Simon prepared styrene by distilling it from storax and called it styrol. Simon observed it solidifi ed into a rubbery substance after being stored and believed it had oxidized into styrol oxide. Subsequent analysis showed the solid did not contain oxygen and it was renamed metastyrol. This was the first written record of polymerization in chemistry. In 1845, the English chemist, John Blyth, and the German chemist, August Wilhelm von Hofmann (1818 1892), observed that styrene was converted to polystyrene by sunlight and that styrene could be polymerized to polystyrene by heating in the absence of oxygen. It took another 70 years for the polymerization of styrene to be described by Hermann Staudinger (1881 1965) in the 1920s. This laid the foundation for the commercial polystyrene industry that developed in the 1930s.
Styrene (monomer) is a viscous, highly flammable liquid that evaporates easily and polymerizes readily to polystyrene unless a stabilizer is added. Styrene is used in multiple industries, especially in reinforced plastics (e.g., fiberglass boats), and is widely used to make plastics and rubber, packaging materials (e.g., packing peanuts ), insulation for buildings, plastic pipes, food containers (e.g., takeout containers), and carpet backing) (ATSDR, 2010).
Safety
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H226-H304-H315-H319-H332-H335-H361-H372-H412 |
| Precautionary statements | P210-P273-P301+P310-P303+P361+P353-P304+P340+P312-P331 |
| Hazard Codes | Xn,T,F |
| Risk Statements | 10-20-36/38-40-36/37/38-39/23/24/25-23/24/25-11-48/20-63 |
| Safety Statements | 23-36-26-16-45-36/37-7-46 |
| OEB | A |
| OEL | TWA: 50 ppm (215 mg/m3), STEL: 100 ppm (425 mg/m3) |
| RIDADR | UN 2055 3/PG 3 |
| WGK Germany | 2 |
| RTECS | WL3675000 |
| Autoignition Temperature | 914 °F |
| TSCA | Yes |
| HS Code | 2902 50 00 |
| HazardClass | 3 |
| PackingGroup | III |
| Hazardous Substances Data | 100-42-5(Hazardous Substances Data) |
| Toxicity | LD50 in mice (mg/kg): 660 ± 44.3 i.p.; 90 ± 5.2 i.v. |
| IDLA | 700 ppm |