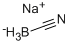Sodium cyanoborohydride , 95% , 25895-60-7
Synonym(s):
Sodium borocyanohydride;Sodium cyanoborohydride;Sodium cyanotrihydridoborate;Sodium cyanotrihydroborate
CAS NO.:25895-60-7
Empirical Formula: CH3BNNa
Molecular Weight: 62.84
MDL number: MFCD00003516
EINECS: 247-317-2
| Pack Size | Price | Stock | Quantity |
| 5G | RMB43.20 | In Stock |
|
| 25G | RMB140.00 | In Stock |
|
| 100G | RMB523.20 | In Stock |
|
| 250G | RMB1263.20 | In Stock |
|
| 500G | RMB1857.60 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | >242 °C (dec.) (lit.) |
| Boiling point: | 307°C |
| Density | 1.083 g/mL at 25 °C |
| Flash point: | −1 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in water (100 mg/ml, with heating), methanol, ethanol, and THF. It is insoluble in nonpolar solvents such as benzene or hexane |
| form | Powder |
| color | White |
| Specific Gravity | 1.2 |
| biological source | synthetic |
| Water Solubility | 2120 g/L at 29 ºC (dec.) |
| Sensitive | Moisture Sensitive |
| Merck | 14,8606 |
| BRN | 4152656 |
| Stability: | Stable. Hygroscopic. Reacts violently with water, giving off and igniting hydrogen. Do not use water on fires involving this chemical - instead use dry soda ash. Incompatible with strong acids, water, strong oxidizing agents. |
| CAS DataBase Reference | 25895-60-7(CAS DataBase Reference) |
| EPA Substance Registry System | Borate(1-), (cyano-.kappa.C)trihydro-, sodium, (T-4)- (25895-60-7) |
Description and Uses
Sodium cyanoborohydride (NaBH3CN) is a selective reducing agent used for a variety of chemical reductions, including aldehyde, ketones, acetals, epoxides, oximes, enamines, reductive aminations of aldehydes and ketones, and reductive alkylations of amines and hydrazines. The utility of sodium cyanoborohydride as a reducing agent is greatly enhanced by its stability under acid conditions, and its solubility in aprotic solvents. Sodium cyanoborohydride is a milder and more selective reducing agent than sodium borohydride.
Sodium cyanoborohydride is a weaker and more-selective reducing agent than sodium borohydride because of the electron-withdrawing effect of the cyano group. It has the further advantage that it is stable in acid to pH = 3 and can be employed to effect reductions in the presence of functional groups that are sensitive to the more-basic conditions of reduction with sodium borohydride.
Aldehydes and ketones are unaffected by sodium cyanoborohydride in neutral solution, but they are readily reduced to the corresponding alcohol at pH=3-4 by way of the protonated carbonyl group. By previous exchange of the hydrogens of the borohydride for deuterium or tritium, by reaction with D2O or tritiated water, an efficient and economical route is available for deuteride or tritiide reduction of aldehydes and ketones.
Sodium Cyanoborohydride is a commonly used as a reagent in the reductive amination of aldehydes and ketones and in the reductive alkylation of amines. It is also used in the synthesis of a novel phenolate-bridged dilanthanum(III) complex of interest as a model for metalloproteins as well as for its importance in basic and applied chemistry.
Safety
| Symbol(GHS) |     GHS02,GHS05,GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H228-H260-H300+H310+H330-H314-H410 |
| Precautionary statements | P210-P231+P232-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | T+,N,T,F |
| Risk Statements | 26/27/28-32-34-50/53-16-15-11-51/53-36-23/24/25-19-14-40-36/37/38 |
| Safety Statements | 26-28-36/37/39-45-60-61-8-50A-43-28A-1-16-36/37 |
| RIDADR | UN 3179 4.1/PG 2 |
| WGK Germany | 1 |
| F | 10-21 |
| Hazard Note | Toxic/Highly Flammable |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | I |
| HS Code | 28500020 |