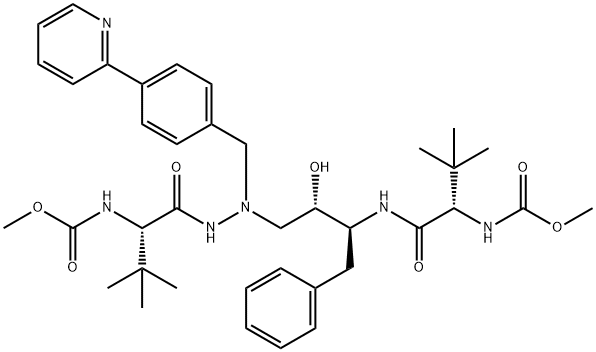
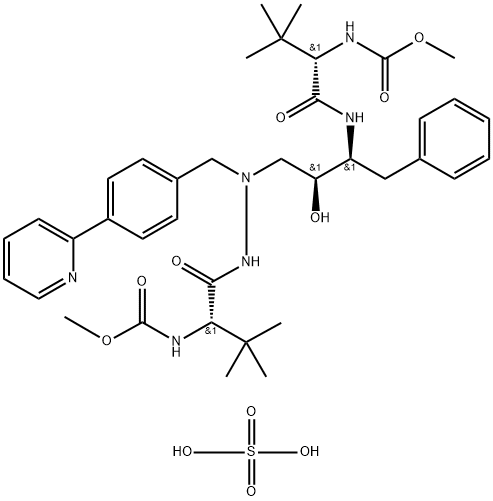
Atazanavir , 10mMinDMSO , 198904-31-3
Synonym(s):
1,14-Dimethyl (3S,8S,9S,12S)-3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pentaazatetradecanedioate;BMS-232632
CAS NO.:198904-31-3
Empirical Formula: C38H52N6O7
Molecular Weight: 704.86
MDL number:
EINECS: 812-543-8
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB159.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 207-2090C |
| alpha | D -47° (c = 1 in ethanol) |
| Density | 1.178±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Ethanol (Slightly), Methanol (Slightly) |
| pka | 11.11±0.46(Predicted) |
| form | powder |
| color | white to beige |
| optical activity | [α]/D -41 to -49°, c =0.1 in ethanol |
| CAS DataBase Reference | 198904-31-3 |
Description and Uses
Atazanavir is an inhibitor of human immunodeficiency virus type 1 (HIV-1) protease, an enzyme that is essential for the processing of Gag and Gag-Pol polyproteins into structural and enzymatic proteins required for viral replication. It has a similar pharmacophore motif to the other six widely marketed HIV protease inhibitors, most of which are based upon a hydroxyethylamine template. Uniquely, it possesses an aza-peptide motif but maintains many similar pharmacophore elements including lipophilic moieties that presumably bind to S2, S1, S′1 , and S′2 positions. Atazanavir is pseudo-symmetric about the central template, incorporating D-tert-Leucine at both termini. This compound is synthesized in about seven steps, with a key coupling of the chiral epoxide (derived from phenylalanine and imparting one chiral center) and N-tert-boc-N′-(4-[2-pyridyl]benzyl)hydrazine. Removal of both tert-Boc groups and double acylation with methoxycarbonyl-tert-Leucine provides the product. Another synthesis of atazanavir entails ten steps and utilizes α-(tert-bocamino) phenylpropanal as a chiral intermediate. It is a potent inhibitor of indinavir-resistant and saquinavir-resistant strains of HIV-1 (IC50=0.03–0.1 and 0.04–0.1 μM, respectively). In 300 patients who had failed previous treatment, atazanavir (400 mg once daily) was compared to lopinavir (400 mg twice daily) and ritonavir (100 mg); both arms additionally receiving two non-reverse transcriptase inhibitors. After 24 weeks, HIV RNA levels of <400 copies/mL were noted in 61% of patients receiving atazanavir and 81% of those taking lopinavir/ritonavir. After 96 weeks of therapy with atazanavir, HIV RNA copy levels were found to be <400 and <50 in 80 and 58% of patients, respectively. A study of the cross-resistance profile relative to other protease inhibitors using a panel of 551 clinical isolates (without prior atazanavir exposure but with cross-resistance to one or two other protease inhibitors; the majority had resistance to nelfinavir) showed that greater than 80% retained susceptibility to atazanavir. All of the resistant isolates from patients taking atazanavir had an I50 L substitution. The recommended dosage of atazanavir is 400 mg once daily. It has a mean half-life range of 7.9–6.5 h with about 60% bioavailability and moderate plasma protein binding (86% albumin and 89% alpha-1- acid glycoprotein (AAG)). Atazanavir was well tolerated in clinical studies and it displayed minimal lipid modulation when tested in combination with two non-reverse transcriptase inhibitors. Atazanavir had no effect on total cholesterol, low-density lipoprotein, and triglyceride levels when compared with other protease inhibitors that caused sustained elevations in these lipid levels.
enzyme inhibitor
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H319 |
| Precautionary statements | P305+P351+P338 |
| Hazardous Substances Data | 198904-31-3(Hazardous Substances Data) |