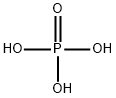
Phosphoric acid , AR,≥85wt.%inH2O , 7664-38-2
Synonym(s):
o-Phosphoric acid, Orthophosphoric acid;Orthophosphoric acid;Orthophosphoric acid, o-Phosphoric acid;Phosphoric acid;Phosphoric acid solution
CAS NO.:7664-38-2
Empirical Formula: H3O4P
Molecular Weight: 98
MDL number: MFCD00011340
EINECS: 231-633-2
PRODUCT Properties
| Melting point: | ~40 °C(lit.) |
| Boiling point: | 158 °C(lit.) |
| Density | 1.685 g/mL at 25 °C(lit.) |
| vapor density | 3.4 (vs air) |
| vapor pressure | 2.2 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2900 | PHOSPHORIC ACID |
| storage temp. | no restrictions. |
| solubility | H2O: soluble |
| pka | 2.1-7.2-12.3(at 25℃) |
| form | Solid or Viscous Liquid |
| Specific Gravity | 1.7 |
| color | ≤10(APHA) |
| PH | 3.06(1 mM solution);2.26(10 mM solution);1.63(100 mM solution); |
| Odor | Odorless |
| PH Range | 1.5 |
| biological source | synthetic |
| Water Solubility | MISCIBLE |
| λmax | λ: 260 nm Amax: ≤0.05 λ: 280 nm Amax: ≤0.04 |
| Merck | 14,7344 |
| BRN | 1921286 |
| Exposure limits | TLV-TWA 1 mg/m3 (ACGIH, MSHA, and
OSHA); TLV-STEL 3 mg/m3 (ACGIH). |
| Dielectric constant | 61.0 |
| InChIKey | NBIIXXVUZAFLBC-UHFFFAOYSA-N |
| LogP | -2.15 |
| CAS DataBase Reference | 7664-38-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphoric acid(7664-38-2) |
| EPA Substance Registry System | Phosphoric acid (7664-38-2) |
Description and Uses
Phosphoric acid was prepared first by Robert Boyle in 1694 by dissolving phosphorus pentoxide in water. Phosphoric acid is probably the most important compound of phosphorus. It is the second largest inorganic chemical by volume, after sulfuric acid, marketed in the United States.
The single most important application of Phosphoric acid is manufacturing phosphate salts for fertilizers. Such fertilizer phosphates include sodium, calcium, ammonium, and potassium phosphates. Other applications are in metal pickling and surface treatment for removal of metal oxides from metal surfaces; electropolishing of aluminum; as a bonding agent in various refractory products such as alumina and magnesia; as a catalyst in making nylon and gasoline; as a dehydrating agent; in fireproofing wood and fabrics; in lithographic engraving; in textile dyeing; in dental cement; in coagulating rubber latex; in purifying hydrogen peroxide; and as a laboratory reagent. Dilute solutions of phosphoric acid are used as additives to carbonated beverages for a pleasing sour taste. Also, dilute acid is used in refining sugar; as a nutrient; and as a buffering agent in preparing jam, jelly, and antibiotics. The commercial phosphoric acid is 85% (w/w) in strength.
In the manufacture of superphosphates for fertilizers, other phosphate salts, polyphosphates, detergents. Acid catalyst in making ethylene, purifying hydrogen peroxide. As acidulant and flavor, synergistic antioxidant and sequestrant in food. Pharmaceutic aid (solvent). In dental cements; process engraving; rustproofing of metals before painting; coagulating rubber latex; as analytical reagent.
Safety
| Symbol(GHS) |   GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H290-H302-H314 |
| Precautionary statements | P234-P270-P280-P301+P312-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | C,Xn,T,F |
| Risk Statements | 34-35-22-39/23/24/25-36/38-23/24/25-11 |
| Safety Statements | 7-16-26-36/37-45-36/37/39-1/2-24/25 |
| RIDADR | UN 3453 8/PG 3 |
| OEB | C |
| OEL | TWA: 1 mg/m3, STEL: 3 mg/m3 |
| WGK Germany | 3 |
| RTECS | TB6300000 |
| F | 3-10 |
| TSCA | Yes |
| HS Code | 2809 20 00 |
| HazardClass | 8 |
| PackingGroup | III |
| Hazardous Substances Data | 7664-38-2(Hazardous Substances Data) |
| Toxicity | ADI 0 to 70 mg / kg (total phosphate content in terms of phosphorus, FAO / WHO, 2001). GRAS (FDA, § 182.1073, 2000). LD501530mg / kg (rat, oral). In case of daily intake of 2 ~ 4 g, it can cause mild diarrhea. The amount of sour agent used as a cola drink is 0.02% to 0.06%. |
| IDLA | 1,000 mg/m3 |