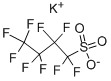
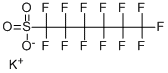
Potassium nonafluoro-1-butanesulfonate , 97% , 29420-49-3
Synonym(s):
Nonafluoro-1-butanesulfonic acid potassium salt;Nonafluorobutane-1-sulfonic acid potassium salt;Potassium nonaflate;Potassium nonafluoro-1-butanesulfonate
CAS NO.:29420-49-3
Empirical Formula: C4F9KO3S
Molecular Weight: 338.19
MDL number: MFCD00007515
EINECS: 249-616-3
| Pack Size | Price | Stock | Quantity |
| 5G | RMB24.00 | In Stock |
|
| 25G | RMB51.20 | In Stock |
|
| 100G | RMB158.40 | In Stock |
|
| 500g | RMB759.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | >300 °C (lit.) |
| Density | 0.69 |
| bulk density | 1000kg/m3 |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Store below +30°C. |
| solubility | water: soluble100mg/mL, clear, colorless |
| form | Solid |
| color | White to Off-White |
| PH | 5.5-6.5 (50g/l, H2O) |
| Water Solubility | water: soluble 100mg/mL, clear, colorless |
| BRN | 5200982 |
| Boiling point: | >400°C |
| Stability: | Very Hygroscopic |
| InChIKey | LVTHXRLARFLXNR-UHFFFAOYSA-M |
| LogP | -1.8 at 23℃ and pH5.9-6.3 |
| Surface tension | 70.45mN/m at 1g/L and 20℃ |
| Dissociation constant | -13.2--12.5 at 22-37℃ |
| CAS DataBase Reference | 29420-49-3(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Butanesulfonic acid, 1,1,2,2,3,3,4,4,4-nonafluoro-, potassium salt (29420-49-3) |
Description and Uses
Potassium nonafluoro-1-butanesulfonate is a chemical compound that is used to remove trifluoroacetic acid from wastewater. It can also be used as an analytical reagent to measure cytosolic calcium concentrations in cells. In addition, it has been shown to have a high degree of chemical stability, but it may react with hydrogen fluoride and cause toxicity in humans.
Potassium nonafluoro-1-butanesulfonate (KFBS), a potassium salt of perfluorobutyl sulfonic acid, is a white crystalline solid with high thermal stability, low vapor pressure, and good solubility in polar solvents. It could be used to synthesize ionic liquids in cooperation with pyrrolidinium salts. These ionic liquids modify the surface of the cathode materials in lithium batteries with enhanced stability and performance. This multifunctional surfactant could be introduced into the SnO2 ETLs. The nonafluorocarbon alkyl chains of KFBS molecule bond with undercoordinated Sn2+ ions in SnO2, which effectively inhibits the charge recombination caused by defects related to oxygen vacancies; the potassium ion (K+) could ameliorate the crystal quality and increase the size of grains of perovskite, adjust the energy level matching between SnO2 and perovskite; the sulfonate group could interact with uncoordinated Pb2+ ions to reduce the surface defects and suppress carrier nonradiative recombination. The power conversion efficiencies increase from 20.6% for pristine devices to 23.21% for KFBS-modified devices[1].
Safety
| Symbol(GHS) |  GHS05 |
| Signal word | Danger |
| Hazard statements | H318 |
| Precautionary statements | P280-P305+P351+P338 |
| Hazard Codes | Xi,C |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| HazardClass | IRRITANT |
| HS Code | 29049090 |
| Toxicity | LD50 orally in Rabbit: > 2000 mg/kg LD50 dermal Rabbit > 2000 mg/kg |