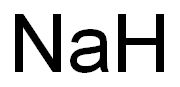Potassium , 99% , 7440-09-7
Synonym(s):
K 006100;Potassium chloride solution
CAS NO.:7440-09-7
Empirical Formula: K
Molecular Weight: 39.1
MDL number: MFCD00133776
EINECS: 231-119-8
PRODUCT Properties
| Melting point: | 64 °C (lit.) |
| Boiling point: | 760 °C (lit.) |
| Density | 0.86 g/mL at 25 °C (lit.) |
| vapor pressure | 0.09 mm Hg ( 260 °C) |
| refractive index | n |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | rod |
| color | Silver/gray |
| Specific Gravity | 0.86 |
| PH | 5.0 (H2O, 20°C) |
| Odor | Odorless |
| Flame Color | Lilac or pale violet |
| Resistivity | 6.1 μΩ-cm, 20°C |
| Water Solubility | reacts |
| Sensitive | Air & Moisture Sensitive |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Dielectric constant | 5(0.0℃) |
| Stability: | Stable. Moisture and air-sensitive. Spontaneously combustible through the generation and ignition of hydrogen. Reacts violently with water and acids, alcohols, carbon monoxide. Store under oil. |
| CAS DataBase Reference | 7440-09-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Potassium(7440-09-7) |
| EPA Substance Registry System | Potassium (7440-09-7) |
Description and Uses
Potassium has atomic number 19 and the chemical symbol K, which is derived from its Latin name kalium . Potassium was first isolated from potash, which is potassium carbonate (K2CO3). Potassium occurs in nature only in the form of its ion (K+) either dissolved in the ocean or coordinated in minerals because elemental potassium reacts violently with water . Potassium ions are essential for the human body and are also present in plants. The major use of K+ can be found in fertilisers, which contains a variety of potassium salts such as potassium chloride (KCl), potassium sulfate (K2SO4) and potassium nitrate (KNO3).
Some of the most common compounds in 19th century photography were made with this silvery metallic element discovered by Sir Humphrey Davy in 1807. There is not enough room in this work to list all of these compounds, but the following represent a reasonable sampling.
Safety
| Symbol(GHS) |   GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H260-H314 |
| Precautionary statements | P223-P231+P232-P260-P280-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | F,C,Xi,T |
| Risk Statements | 14/15-34-36/38-23/24/25 |
| Safety Statements | 8-43-45-5B-5*-36/37/39-26-5-27 |
| RIDADR | UN 2257 4.3/PG 1 |
| WGK Germany | 2 |
| RTECS | TS8050000 |
| F | 8 |
| Autoignition Temperature | 25 °C or below in air or oxygen |
| TSCA | Yes |
| HS Code | 2827 39 85 |
| HazardClass | 4.3 |
| PackingGroup | I |
| Hazardous Substances Data | 7440-09-7(Hazardous Substances Data) |
| Toxicity | Ignites in air and reacts explosively with water; highly corrosive to the skin and eyes. Potassium reacts with the moisture on skin and other tissues to form highly corrosive potassium hydroxide. Contact of metallic potassium with the skin, eyes, or mucous membranes causes severe burns; thermal burns may also occur due to ignition of the metal and liberated hydrogen. |