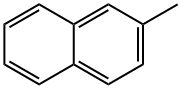
2-Methylnaphthalene , Analyze the standard products for environmental analysis , 91-57-6
Synonym(s):
β-Methylnaphthalene
CAS NO.:91-57-6
Empirical Formula: C11H10
Molecular Weight: 142.2
MDL number: MFCD00004118
EINECS: 202-078-3
PRODUCT Properties
| Melting point: | 34-36 °C(lit.) |
| Boiling point: | 241-242 °C(lit.) |
| Density | 1.01 |
| vapor pressure | 5.4 (extrapolated, Mackay et al., 1982) |
| refractive index | 1.6019 |
| Flash point: | 208 °F |
| storage temp. | 2-8°C |
| solubility | 0.025g/l |
| form | Crystalline Low Melting Solid |
| color | White |
| Odor | at 1.00 % in dipropylene glycol. sweet floral woody |
| Odor Type | floral |
| Water Solubility | 0.00246 g/100 mL |
| FreezingPoint | 34.5℃ |
| BRN | 906859 |
| Henry's Law Constant | 6.13 at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Exposure limits | ACGIH: TWA 0.5 ppm (Skin) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| Cosmetics Ingredients Functions | PERFUMING |
| InChIKey | QIMMUPPBPVKWKM-UHFFFAOYSA-N |
| LogP | 3.860 |
| CAS DataBase Reference | 91-57-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Naphthalene, 2-methyl-(91-57-6) |
| EPA Substance Registry System | 2-Methylnaphthalene (91-57-6) |
Description and Uses
2-Methylnaphthalene is a polycyclic aromatic hydrocarbon (PAH), consisting of two-fused aromatic rings with a methyl group attached on one of the rings at the number two carbon.
2-Methylnaphthalene is a natural component of crude oil and coal, and is found in pyrolysis and combustion products such as cigarette and wood smoke, emissions from combustion engines, asphalt, coal tar residues, and used oils (ATSDR, 1995; HSDB, 2002; Warshawsky, 2001).
In the United States, 2-methylnaphthalene is used for making detergents, dyes, solvents, as well as vitamin K. 2-methylnapthalene is also used to make some pesticides, or as an additional ingredient in some pesticides. 2- methylnaphthalene is released into the environment when wood or fossil fuels are burned or when there are spills of products containing fossil fuels. 2-methylnaphthalene can evaporate or break down quickly in soils exposed to air or containing certain microorganisms, but it could stay a year or more under other conditions in certain sediments or soils.
2-methylnaphthalene are used to make other chemicals such as dyes, insecticides, Organic synthesis and resins. Pure 2-methylnaphthalene is a component used in the manufacture of vitamin K and the insecticide carbaryl (1-naphthyl-N-methylcarbamate) (HSDB, 2002).
Methylnaphthalene (CASRN 1321-94-4) refers to a mixture of approximately two-thirds 2-methylnaphthalene and one-third 1-methylnaphthalene (CASRN 90-12-0). Methylnaphthalene is manufactured from coal tar through the extraction of heteroaromatics and phenols. Distillation of methylnaphthalene removes 1-methylnaphthalene, leaving 2-methylnaphthalene. Mixtures containing 2-methylnaphthalene are used in the formulation of alkyl-naphthalenesulfonates (used for detergents and textile wetting agents), chlorinated naphthalenes, and hydronaphthalenes (used as solvents).
Safety
| Symbol(GHS) |   GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335-H411 |
| Precautionary statements | P261-P264-P273-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | F,Xn,N |
| Risk Statements | 22-36/37/38-51/53-63-43-23/24/25-45-20/21/22-67-40 |
| Safety Statements | 26-37/39-61-36/37-24/25-23-53-36-29 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 2 |
| RTECS | QJ9635000 |
| TSCA | Yes |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 29029080 |
| Hazardous Substances Data | 91-57-6(Hazardous Substances Data) |
| Toxicity | Acute oral LD50 for rats 1,630 mg/kg (quoted, RTECS, 1985). |