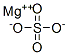Magnesium sulfate , ≥99.5% , 7487-88-9
Synonym(s):
Magnesium sulfate anhydrous;Magnesium Sulfate, Anhydrous;Sulfuric Acid Magnesium Salt, Epsom salts
CAS NO.:7487-88-9
Empirical Formula: MgSO4
Molecular Weight: 120.37
MDL number: MFCD00011110
EINECS: 231-298-2
| Pack Size | Price | Stock | Quantity |
| 100g | RMB31.20 | In Stock |
|
| 500G | RMB71.20 | In Stock |
|
| 2.5KG | RMB239.20 | In Stock |
|
| 10KG | RMB719.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 1124 °C |
| Density | 1.07 g/mL at 20 °C |
| bulk density | 600kg/m3 |
| vapor density | <0.01 (vs air) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| storage temp. | no restrictions. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | powder (very fine) |
| Specific Gravity | 2.66 |
| color | slightly gray |
| Odor | at 100.00 %. odorless |
| PH | 7.9 (50g/l, H2O, 25℃) |
| Odor Type | odorless |
| Water Solubility | Soluble in water. Slightly soluble in alcohol, glycerol. Insoluble in acetone. |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,5691 |
| Dielectric constant | 8.2(Ambient) |
| Stability: | Stable. Hygroscopic. |
| LogP | -1.031 (est) |
| CAS DataBase Reference | 7487-88-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium sulfate(7487-88-9) |
| EPA Substance Registry System | Magnesium sulfate (7487-88-9) |
Description and Uses
Magnesium sulfate is an inorganic salt (chemical compound) containing magnesium, sulfur and oxygen, with the formula MgSO4. It is strongly hygroscopic and often encountered as the heptahydrate sulfate mineral epsomite (MgSO4•7H2O), commonly called Epsom salt. And the monohydrate, MgSO4•H2O, is found as the mineral kieserite.
In gardening and other agriculture, magnesium sulfate is used to correct a magnesium or sulfur deficiency in soil.
In food preparation, magnesium sulfate is used as a brewing salt in beer production or used as a coagulant for making tofu.
In chemistry, anhydrous magnesium sulfate is commonly used as a desiccant in organic synthesis due to its affinity for water.
For Marine use, magnesium sulfate heptahydrate is used to maintain the magnesium concentration in marine aquaria which contain large amounts of stony corals.
For medicine use, it is used in pregnant women to control seizures due to certain complications of pregnancy (eg, severe toxemia) and to control high blood pressure, severe brain function problems (encephalopathy), and seizures in children who have sudden, severe inflammation of the kidneys (acute nephritis). Besides, magnesium sulfate is also used as a laxative to relieve occasional constipation.
In agriculture and gardening, magnesium sulfate is
used to correct magnesium deficiency in soil, since
magnesium is an essential element in the chlorophyll
molecule. It is most commonly applied to potted
plants, or to magnesium-hungry crops, such as potatoes,
tomatoes, peppers, and roses. The advantage of
magnesium sulfate over other magnesium compounds
(such as dolomitic limestone) is its high solubility.
MgSO4 has been used in organic synthesis to remove
water from nonaqueous solutions before the organic
reaction is started. Since it is insoluble in most organic
solvents, its addition forms hydrates that can be easily
removed.
Epsom salt is also used to prepare footbaths,
intended to soothe sore feet. The reason for the inclusion
of the salt is partially cosmetic. However, magnesium
sulfate can also be absorbed into the skin,
reducing inflammation. It is also sometimes found in
bottled mineral water, and accordingly is sometimes
listed in the contents thereof. Magnesium sulfate heptahydrate
is also used to maintain the magnesium concentration
in marine aquaria which contain large amounts
of stony corals as it is slowly depleted in their calcification
process, precipitation into calcium carbonate. Oral
Epsom salt is used as a saline laxative as well as for
replacement therapy in “hypomagnesaemia” (lack of
Mg2+) in animals and humans. Magnesium sulfate
paste has been used as an agent for dehydrating
(drawing) boils, carbuncles or abscesses. Magnesium
sulfate solution has also been shown to be an effective
aid in the fight against blemishes and acne when
applied directly to problematic areas. Magnesium
sulfate, when used through soaking, can soothe muscle pains and help improve rough patches in the skin. Soaking
in a warm bath containing Epsom salt (magnesium
sulfate) can be beneficial to soothe and relieve herpes
outbreak symptoms, such as itching and lesions relating
to genital herpes or shingles.
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Warning |
| Hazard statements | H371 |
| Precautionary statements | P501-P260-P270-P264-P308+P311-P405 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 22-24/25-36-26 |
| WGK Germany | 1 |
| RTECS | OM4500000 |
| F | 3 |
| TSCA | Yes |
| HS Code | 28332100 |
| Hazardous Substances Data | 7487-88-9(Hazardous Substances Data) |