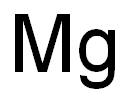Magnesium standard , 100ug/mlin5%HCl , 7439-95-4
Synonym(s):
Magnesium;NMDG;Magnesium foil;Magnesium Shot;Magnesium element
CAS NO.:7439-95-4
Empirical Formula: Mg
Molecular Weight: 24.31
MDL number: MFCD00085308
EINECS: 231-104-6
PRODUCT Properties
| Melting point: | 648 °C (lit.) |
| Boiling point: | 1090 °C (lit.) |
| Density | 1.74 g/mL at 25 °C (lit.) |
| vapor density | 6 (vs air) |
| vapor pressure | 1 mm Hg ( 621 °C) |
| Flash point: | −26 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | turnings |
| color | White |
| Specific Gravity | 1.74 |
| Flame Color | White sparks |
| Resistivity | 4.46 μΩ-cm, 20°C |
| Water Solubility | REACTS |
| Sensitive | Hygroscopic |
| Crystal Structure | HCP, Space Group P63/mmc |
| Merck | 14,5674 |
| BRN | 4948473 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| LogP | -0.57 at 20℃ |
| CAS DataBase Reference | 7439-95-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium(7439-95-4) |
| EPA Substance Registry System | Magnesium (7439-95-4) |
Description and Uses
Magnesium is a Group 2 element (Group IIA in older labeling schemes). This element has the symbol Mg, atomic number 12, atomic weight of 24.305 g/mol and common oxidation number +2. It is the eighth most abundant element in the earth s crust by mass, although ninth in the Universe as a whole. This preponderance of magnesium in the Universe is related to the fact that it is easily built up in supernova stars from a sequential addition of three helium nuclei to carbon (which in turn is made from three helium nuclei). Magnesium constitutes about 2% of the Earth s crust by mass, which makes it the eighth most abundant element in the crust. Magnesium ion’s high solubility in water helps to ensure that it is the third most abundant element dissolved in seawater.
In alloys to produce light weight structural metals. In aluminum alloys to improve mechanical properties; in Grignard reagents; in dry cell batteries; in pyrotechnics. For hot metal desulfurization, especially. molten iron; production of ductile iron; metal reduction to produce elemental boron, titanium, zirconium; corrosion protection of steel structures; sacrificial anodes for corrosion protection.
Safety
| Symbol(GHS) |  GHS02 |
| Signal word | Danger |
| Hazard statements | H228-H261 |
| Precautionary statements | P210-P223-P231+P232-P240-P241-P280 |
| Hazard Codes | F,Xn |
| Risk Statements | 34-15-11-17-36/37/38-22-19-40-36/37 |
| Safety Statements | 43-7/8-43A-36-33-26-36/37-16 |
| RIDADR | UN 2056 3/PG 2 |
| WGK Germany | 1 |
| RTECS | OM3756000 |
| F | 3-9 |
| Autoignition Temperature | 950 °F |
| TSCA | Yes |
| HazardClass | 4.1 |
| PackingGroup | III |
| HS Code | 81049000 |
| Hazardous Substances Data | 7439-95-4(Hazardous Substances Data) |