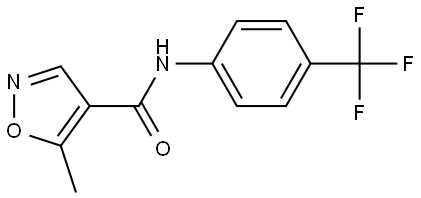
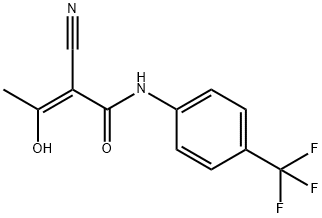
Leflunomide , ≥98% , 75706-12-6
Synonym(s):
5-Methyl-N-[4-(trifluoromethyl) phenyl]-isoxazole-4-carboxamide;5-Methylisoxazole-4-(4-trifluoromethyl)carboxanilide;Leflunomide
CAS NO.:75706-12-6
Empirical Formula: C12H9F3N2O2
Molecular Weight: 270.21
MDL number: MFCD00867593
EINECS: 616-254-6
| Pack Size | Price | Stock | Quantity |
| 50MG | RMB35.20 | In Stock |
|
| 250MG | RMB120.80 | In Stock |
|
| 1G | RMB263.20 | In Stock |
|
| 200mg | RMB712.00 | In Stock |
|
| 5g | RMB758.40 | In Stock |
|
| 25g | RMB1945.60 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 166.5 °C |
| Boiling point: | 289.3±40.0 °C(Predicted) |
| Density | 1.392±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in methanol, sparingly soluble in methylene chloride. |
| form | Solid |
| pka | 10.8(at 25℃) |
| color | White |
| Merck | 14,5432 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
| InChIKey | VHOGYURTWQBHIL-UHFFFAOYSA-N |
| CAS DataBase Reference | 75706-12-6(CAS DataBase Reference) |
Description and Uses
Leflunomide is an orally-available disease-modifying antirheumatic drug
and was launched as Arava in the US for the treatment of rheumatoid arthritis
(RA) ; it is the first and only drug to be indicated to slow down structural joint
damage of RA, so addressing an unmet medical need.
Leflunomide is prepared in 3 steps from the appropriate acetoacetic anilide
using a nitrile oxide- enamine cycloaddition reaction to assemble the isoxazole
ring. Leflunomide is a prodrug, being extensively metabolized in vivo into the
corresponding 2-cyano-3-hydroxy-2-butenamide resulting from fragmentation of
the isoxazole ring. This cyanoenol is actually the active metabolite and several
experiments in animals have demonstrated that after oral administration,
substantial and sustained levels of this metabolite were delivered to the
systemic circulation.
In vitro, Leflunomide’s active metabolite inhibits dihydroorotate dehydrogenase,
an enzyme involved in the biosynthesis of pyrimidine nucleotides, probably
accounting for its immunosuppressive effect in vivo. Other mechanisms of action
such as inhibition of tyrosine kinase and inhibition of responsiveness to
interleukin-2 have been proposed. In diverse models of autoimmune or allergic
diseases, Leflunornide showed efficacy both prophylactically and therapeutically.
vasodilator
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H301-H315-H319-H335 |
| Precautionary statements | P261-P264-P270-P301+P310-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | NY2354200 |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 2934990002 |
| Hazardous Substances Data | 75706-12-6(Hazardous Substances Data) |