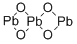Lead oxide red , 99.95%metalsbasis , 1314-41-6
Synonym(s):
Lead oxide, red;Lead(II,IV) oxide;Minium;Red lead oxide;Trilead tetraoxide
CAS NO.:1314-41-6
Empirical Formula: O4Pb3
Molecular Weight: 685.6
MDL number: MFCD00078491
EINECS: 215-235-6
PRODUCT Properties
| Melting point: | 500 °C |
||||||||||||||
| Boiling point: | 800°C |
||||||||||||||
| Density | 9,1 g/cm3 |
||||||||||||||
| vapor pressure | 10 mm Hg ( 0 °C) |
||||||||||||||
| solubility | insoluble in H2O, ethanol; soluble in hotHCl |
||||||||||||||
| form | red powder |
||||||||||||||
| color | Orange |
||||||||||||||
| Water Solubility | Soluble in hydrochloric acid, glacial acetic acid and nitric acid and hydrogen peroxide. Insoluble in water and alcohol. |
||||||||||||||
| Merck | 14,5425 |
||||||||||||||
| crystal system | square |
||||||||||||||
| Space group | P42/mbc |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
||||||||||||||
| Dielectric constant | 25.9(0.0℃) |
||||||||||||||
| Stability: | Stable. May react vigorously with reducing agents. |
||||||||||||||
| InChIKey | XMFOQHDPRMAJNU-UHFFFAOYSA-N |
||||||||||||||
| CAS DataBase Reference | 1314-41-6(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Lead(ii,iv) oxide(1314-41-6) |
||||||||||||||
| EPA Substance Registry System | Lead tetraoxide (1314-41-6) |
Description and Uses
Lead oxide, a range of products that is formed by the oxidation of Lead in the forms of liquid and solid. Lead oxides are basically an oxide’s family varying in color (grey/green, red, and yellow), in degree of oxidation (PbO, Pb3O4, PbO2) and in crystal structure (in forms of PbO, orthogonal and tetragonal).
Lead oxide is a term that can be either Lead monoxide or litharge Lead tetroxide or Red Lead or Gray or Black oxide which is a mixture of 30 percent metallic Lead and 70 percent Lead monoxide. Black Lead is made for specific use in Lead acid storage batteries manufacturing. Due to large use in the Lead acid battery industry, Lead monoxide is one of the most important compounds of Lead, based on volume. Due to its electrical and electronic properties, litharge is also used in various components for different types of use like capacitors, electro photographic plates, and Video tubes, even in ferromagnetic and ferroelectric materials. Their wide range of chemical and physical properties, Lead oxides have been know and used worldwide since before the ancient Romans.
Lead(II,IV) oxide is used to prepare colorless glass, faience glaze, porcelain painting flux, iron and steel coatings, rubber pigment and in glass cement. It is also used in gas and steam pipes, storage batteries, writing on glass and to make lead peroxide and matches. It is associated with linseed oil and used as a thick, long-lasting anti-corrosive paint. It gives better water resistant properties by replacing magnesium oxide. Further, it is used in the manufacture of lead glass and rustproof primer paints. In addition to this, it acts as a pigment for primer paints for iron objects.
Safety
| Symbol(GHS) |     GHS03,GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H272-H302+H332-H351-H360-H372-H410 |
| Precautionary statements | P202-P210-P273-P301+P312-P304+P340+P312-P308+P313 |
| Hazard Codes | O,T,N |
| Risk Statements | 61-8-20/22-33-50/53-62-48/23/25-40 |
| Safety Statements | 53-45-60-61-36/37 |
| RIDADR | UN 1479 5.1/PG 2 |
| WGK Germany | 3 |
| RTECS | OG5425000 |
| TSCA | Yes |
| HazardClass | 5.1 |
| HS Code | 28249010 |
| Hazardous Substances Data | 1314-41-6(Hazardous Substances Data) |
| Toxicity | LD50 i.p. in rats: 45 mg Pb/100 g (Lead, 1972) |