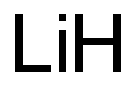Lithium , 99.9%metalsbasis , 7439-93-2
Synonym(s):
;Lithium;Lithium atom;Lithium element;Lithium foil
CAS NO.:7439-93-2
Empirical Formula: Li
Molecular Weight: 6.94
MDL number: MFCD00134051
EINECS: 231-102-5
PRODUCT Properties
| Melting point: | 180 °C (lit.) |
| Boiling point: | 1342 °C (lit.) |
| Density | 0.534 g/mL at 25 °C (lit.) |
| vapor pressure | 1 hPa (723 °C) |
| storage temp. | water-free area |
| solubility | reacts with H2O |
| form | wire |
| Specific Gravity | 0.534 |
| color | Silvery |
| Odor | Odorless |
| PH Range | >12 |
| PH | 5.0 (20°C in H2O) |
| Flame Color | Pink-red or magenta |
| Resistivity | 9.446 μΩ-cm, 20°C |
| Water Solubility | REACTS |
| Sensitive | air sensitive, moisture sensitive |
| Merck | 13,5542 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability: | Stable, but reacts violently with water. |
| LogP | -0.77 at 25℃ |
| Hardness, Vickers | <= 5.0 |
| CAS DataBase Reference | 7439-93-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Lithium(7439-93-2) |
| EPA Substance Registry System | Lithium (7439-93-2) |
Description and Uses
Lithium is an alkali metal with physiologic actions similar to potassium and sodium. Lithium was discovered as a salt in 1817 by Johan August Arfwedson. It does not occur in nature as a free metal but is found in minerals such as spodumene, petalite, and eucryptite. It is the 27th most abundant element in Earth’s crust. Lithium was used to treat gout, as a salt substitute, and as a major constituent of the soft drink 7-Up before 1950.
In production of organometallic alkyl and aryl lithium compounds; in production of high-strength, low-density aluminum alloys for the aircraft industry; extremely tough, low-density alloys with aluminum and magnesium used for armour plate and aerospace components. In polymerization catalysts for the polyolefin plastics industry; manufacture of high-strength glass and glass-ceramics. As anode in electrochemical cells and batteries; as chemical intermediate in organic syntheses. Lithium stearate as thickener and gelling agent to transform oils into lubricating greases.
Safety
| Symbol(GHS) |   GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H260-H314 |
| Precautionary statements | P223-P231+P232-P280-P305+P351+P338-P370+P378-P422 |
| Hazard Codes | Xi,C,F |
| Risk Statements | 36/38-34-14/15-23 |
| Safety Statements | 8-43-45-43C-36/37/39-26 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 2 |
| RTECS | OJ5540000 |
| F | 10 |
| Autoignition Temperature | 179oC |
| TSCA | Yes |
| HS Code | 2805 19 90 |
| HazardClass | 4.3 |
| PackingGroup | I |
| Hazardous Substances Data | 7439-93-2(Hazardous Substances Data) |
| Toxicity | An element used clinically as one of its salts. It is effective against both mania and depression. Despite its effectiveness, there are no clear mechanisms that have been directly related to its therapeutic effectiveness although its inhibition of the formation of inositol from inositol phosphate is thought to be important. At therapeutic concentrations, lithium causes almost no discernible psychotropic effects in healthy humans. The major complaints when the serum concentrations of the drug are carefully monitored include slight muscular weakness, thirst, and excessive urination. The major difficulty with lithium is that a fairly high concentration of the ion is needed in the blood (0.5_x0002_1.0 mmol/L) for maintenance, higher for acute mania. Toxic symptoms (which can involve many physiological symptoms) may occur, however, at doses of 1.5 mmol/L or higher. This low therapeutic index is indicative of the need for regular monitoring of lithium concentrations in the serum. |