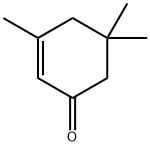
Isophorone , 97% , 78-59-1
Synonym(s):
3,5,5-Trimethyl-2-cyclohexen-1-one;Isophorone
CAS NO.:78-59-1
Empirical Formula: C9H14O
Molecular Weight: 138.21
MDL number: MFCD00001584
EINECS: 201-126-0
| Pack Size | Price | Stock | Quantity |
| 25ML | RMB23.20 | In Stock |
|
| 100ml | RMB35.20 | In Stock |
|
| 250ML | RMB41.60 | In Stock |
|
| 500ML | RMB56.00 | In Stock |
|
| 2.5L | RMB173.60 | In Stock |
|
| 10l | RMB600.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | -8 °C (lit.) |
| Boiling point: | 213-214 °C (lit.) |
| Density | 0.923 g/mL at 25 °C (lit.) |
| vapor density | 4.77 (vs air) |
| vapor pressure | 0.2 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 3553 | ISOPHORONE |
| Flash point: | 184 °F |
| storage temp. | Store below +30°C. |
| solubility | 14.5g/l |
| form | Liquid |
| color | Clear colorless to yellow |
| Odor | Like camphor. |
| explosive limit | 0.8-3.8%(V) |
| Odor Type | woody |
| biological source | synthetic |
| Water Solubility | Soluble in water (12g/L). |
| Merck | 14,5196 |
| JECFA Number | 1112 |
| BRN | 1280721 |
| Henry's Law Constant | (x 10-6 atm?m3/mol):
5.8 (calculated, U.S. EPA, 1980a) |
| Exposure limits | TLV-TWA 25 mg/m3 (5 ppm); IDLH 800
ppm. |
| Stability: | Stable. Substances to be avoided include strong bases, strong acids and strong oxidizing agents. |
| LogP | 1.67-1.7 at 20℃ |
| CAS DataBase Reference | 78-59-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Cyclohexen-1-one, 3,5,5-trimethyl-(78-59-1) |
| EPA Substance Registry System | Isophorone (78-59-1) |
Description and Uses
Isophorone is used as a solvent for vinylresins and cellulose esters, and in pesticides. solvent in some printing inks, paints, lacquers and adhesives. As a cyclic unsaturated ketone, isophorone can be aromatized to 3,5-dimethylphenol or 2,3,5-trimethylphenol. Hydrogenation leads, depending on conditions, to 3,3,5-trimethylcyclohexanone or 3,3,5- trimethylcyclohexanol, from which trimethyladipic acid is obtained by oxidation [78]. Trimethyladipic acid is used for the production of 2,2,4- trimethylhexamethylenediamine, which is a precursor for polyamides and polyurethanes. Addition of hydrogen cyanide to the olefinic double bond, followed by amination of the keto group in the presence of hydrogen, gives 3,5,5-trimethyl-3-aminomethylcyclohexylamine [2855-13-2], isophorone diamine. Reaction of the latter with phosgene gives isophorone diisocyanate [4098-71-9]. Both compounds are used in large quantities for the production of polymers, mainly polyurethanes. Worldwide production capacity for isophorone is ca. 50 000 t/a.
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302+H312-H319-H335-H351 |
| Precautionary statements | P201-P280-P301+P312-P302+P352+P312-P305+P351+P338-P308+P313 |
| Hazard Codes | Xn |
| Risk Statements | 21/22-36/37-40 |
| Safety Statements | 13-23-36/37/39-46 |
| RIDADR | UN 3082 9 / PGIII |
| OEB | A |
| OEL | TWA: 4 ppm (23 mg/m3) |
| WGK Germany | 1 |
| RTECS | GW7700000 |
| Autoignition Temperature | 864 °F |
| TSCA | Yes |
| HS Code | 2914 29 00 |
| Hazardous Substances Data | 78-59-1(Hazardous Substances Data) |
| Toxicity | LD50 in male, female rats and male mice (mg/kg): 2700 ±200, 2100 ±200, 2200 ±200 orally (PB90-180225) |
| IDLA | 200 ppm |