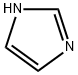
Imidazole , 99% , 288-32-4
Synonym(s):
Imidazole;Glyoxaline;Glyoxaline solution;Glyoxaline, 1;Glyoxaline, Iminazole
CAS NO.:288-32-4
Empirical Formula: C3H4N2
Molecular Weight: 68.08
MDL number: MFCD00005183
EINECS: 206-019-2
| Pack Size | Price | Stock | Quantity |
| 25G | RMB24.00 | In Stock |
|
| 100G | RMB28.80 | In Stock |
|
| 250G | RMB60.80 | In Stock |
|
| 500G | RMB119.20 | In Stock |
|
| 1KG | RMB151.20 | In Stock |
|
| 5KG | RMB639.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 88-91 °C(lit.) |
| Boiling point: | 256 °C(lit.) |
| Density | 1.01 g/mL at 20 °C |
| bulk density | 500-600kg/m3 |
| vapor pressure | <1 mm Hg ( 20 °C) |
| refractive index | 1.4801 |
| Flash point: | 293 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: 0.1 M at 20 °C, clear, colorless |
| form | crystalline |
| pka | 6.953(at 25℃) |
| color | white |
| Specific Gravity | 1.03 |
| Odor | Amine like |
| PH Range | 9.5 - 11 |
| PH | 9.5-11.0 (25℃, 50mg/mL in H2O) |
| Water Solubility | 633 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.10 λ: 280 nm Amax: 0.10 |
| Merck | 14,4912 |
| BRN | 103853 |
| Dielectric constant | 23.0(Ambient) |
| Stability: | Stable. Incompatible with acids, strong oxidizing agents. Protect from moisture. |
| InChIKey | RAXXELZNTBOGNW-UHFFFAOYSA-N |
| LogP | -0.02 at 25℃ |
| CAS DataBase Reference | 288-32-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-Imidazole(288-32-4) |
| EPA Substance Registry System | Imidazole (288-32-4) |
Description and Uses
Imidazole group denotes a heterocyclic organic compound whose molecule contains a five-membered hetero-aromatic ring of two non-adjacent nitrogen atoms, that a carbon atom is placed between two nitrogen atoms. Imidazole is the universally used trivial name for 1,3-azole. Earlier given names were glyoxaline and iminazole. The importance of this aromatic ring system is reflected by its presence in naturally occurring histidine, histamine and the purines, and in several classes of pharmaceuticals. Imidazole can act as a base and as a weak acid. It exists in two tautomeric forms with the hydrogen atom taking position between the two nitrogen atoms.
Imidazole is used as a buffer in the range of pH 6.2-7.8. It is also an histamine antagonist. It acts as a chelator and forms complexes with various divalent cations. It is used as a corrosion inhibitor on certain transition metals such as copper. Its derivatives, like polybenzimidazole (PBI), act as fire retardants. It finds application in photography and electronics. Imidazole salts are used as ionic liquids and precursors to stable carbenes. Imidazole derivatives like ketoconazole, miconazole and clotrimazole are involved in the treatment of various systemic fungal infections. It is a part of the theophylline molecule, present in tea leaves and coffee beans, which stimulates the central nervous system.
Safety
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H302-H314-H360D |
| Precautionary statements | P260-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | C,Xi,T |
| Risk Statements | 36/38-63-34-22-20/21/22-61 |
| Safety Statements | 26-36/37/39-45-22-36-27-53 |
| RIDADR | UN 2923 8/PG 3 |
| WGK Germany | 1 |
| RTECS | NI3325000 |
| Autoignition Temperature | 480 °C |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 29332990 |
| Hazardous Substances Data | 288-32-4(Hazardous Substances Data) |
| Toxicity | LD50 in mice (mg/kg): 610 i.p.; 1880 orally (Nishie) |