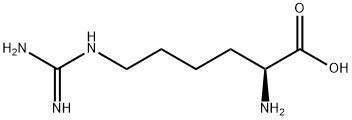
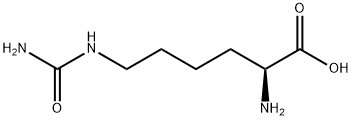
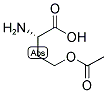
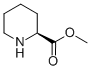
PRODUCT Properties
| Melting point: | 207-209 °C |
| Boiling point: | 376.3±52.0 °C(Predicted) |
| Density | 1.39±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Powder |
| pka | 2.52±0.24(Predicted) |
| color | White to off-white |
| CAS DataBase Reference | 156-86-5(CAS DataBase Reference) |
Description and Uses
Homoarginine and 1-hydroxyhomoarginine were originally detected in significant concentration in the seeds of many Lathyrus species (Bell, 1962a,b; 1963). Homoarginine was isolated from the seeds of L. cicera (Bell, 1962b) and L. sativus (Rao et al., 1963). The hydroxy compound was obtained from the seeds of L. tingitanus (Bell, 1964). The comparative distribution of guanidino compounds in the seeds of two genus of the Papilionoideae, Lathyrus and Vicia, exhibits interesting features (Bell and Tirimanna, 1964). For example, the genus Lathyrus stores predominantly the C7 amino acids homoarginine, y-hydroxyhomoarginine and the related compound lathyrine, while the genus Vicia stores relatively high concentrations of canavanine and of the C6 amino acids arginine and y-hydroxyarginine. Apart from plants, homoarginine was found in small amounts in human body fluids ,. urine (Armstrong, 1967), serum (Matsumoto et al., 1976a) and cerebrospinal fluid (Mori et al., 1981b) and in mammalian brain (Mori et al., 1978, 1979 ). Increased urinary excretion was reported in hyperlysinemia (Armstrong and Robinow, 1967) and citrullinemia (Scott-Emuakpor et al., 19(2).
Homoarginine may be formed in mammals from lysine, by a homologous urea cycle in which arginine is replaced by lysine (Ryan and Wells, 1964; Scott-Emuakpor et al., 19(2). The excretion of homocitrulline and homoarginine in urine following administration of lysine to children supports this theory (Buergi et al., 1966). The biosynthesis of homoarginine has not been examined in plants, but y-hydroxyhomoarginine is synthesized from homoarginine in Lathyrus seeds (Bell, 1964). Subsequently both compounds have been identified as the precursors of the cyclic guanidino derivative, lathyrine, also present in Lathyrus seeds (Bell and Przybylska, 1965). Homoarginine and y-hydroxyhomoarginine probably serve as nitrogen reserves in Lathyrus seeds.
Inhibition of growth by homoarginine has been reported In various microorganisms (Walker, 1955; Rao et al., 1963).
gama-Hydroxyhomoarginine lactone isolated from the seeds of L. tingitanus (Bell, 1964) probably results from the cyclization of the open chain compound under acidic conditions.
Homoarginine is an inhibitor of human arginase activity.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H312-H332 |
| Precautionary statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P330-P363-P501 |