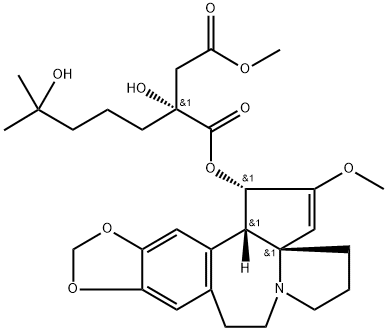
Homoharringtonine , Analysis of standard products, ≥98% , 26833-87-4
Synonym(s):
Omacetaxine mepesuccinate;HHT;Ceflatonin;Cephalotaxine 4-methyl (2R)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate;Myelostat
| Pack Size | Price | Stock | Quantity |
| 20MG | RMB421.60 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 144-146 C |
| Boiling point: | 619.03°C (rough estimate) |
| Density | 1.2395 (rough estimate) |
| refractive index | 1.6290 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| pka | 11.60±0.29(Predicted) |
| form | powder |
| color | white to beige |
| optical activity | [α]/D -120 to -140°, c = 1 in chloroform-d |
| biological source | mouse |
| InChIKey | DJIVDDPFKDEQIR-XSEHADPMSA-N |
Description and Uses
Omacetaxine mepesuccinate (also known as homoharringtonine) was approved by the US FDA in October 2012 for the treatment of patients with chronic or accelerated phase chronic myeloid leukemia (CML) with resistance or intolerance to at least two tyrosine kinase inhibitors (TKIs). Omacetaxine is a protein synthesis inhibitor that was studied in the 1970s for the treatment of acute myeloid leukemia (AML) and in the 1990s for CML. Emergence of resistance to first- and second-generation TKIs has lead to renewed interest in omacetaxine due to its differentiated mode of action. Omacetaxine acts on the initial step of protein translation andresults in the rapid loss of a number of short-lived proteins that regulate proliferation and cell survival. Omacetaxine induces apoptosis and shows in vitro activity in anumberof leukemia cell lines andinmurine leukemiamodels. Omacetaxineis a naturally occurring alkaloid isolated from Cephalotaxus coniferous shrubs that are indigenous to Asia. Extracts of the bark have been used by practitioners of traditional Chinese medicine for the treatment of cancer. Although omacetaxine could be isolated directly from bark and roots, a more efficient approach is semi-synthesis by esterification of the abundant biosynthetic precursor cephalotaxine, which can be extracted from leaves rather than nonrenewable sources. Esterification is carried out with an activated ester in which the diol side-chain is protected as a tetrahydropyran; after ester formation, the diol is released in two steps under mild conditions.
Homoharringtonine has been used to check the cytotoxic activity against carfilzomib-resistant derivative of the LP-1 multiple myeloma (MM) cell line (LP-1/Cfz) and is used as a translation-inhibiting drug.
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H300+H310+H330 |
| Precautionary statements | P262-P280-P301+P310+P330-P302+P352+P310-P304+P340+P310 |
| Hazard Codes | T+,Xi |
| Risk Statements | 26/27/28-36/37/38-28 |
| Safety Statements | 36/37/39-45-27-26-36/37-28 |
| RIDADR | UN 1544 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | FK0260000 |
| HazardClass | 6.1(a) |
| PackingGroup | II |