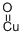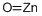Cupric oxide wire , mixtureofCuOandCu2O,ACSreagent , 1317-38-0
Synonym(s):
Copper monoxide granulated;Copper(II) oxide;Cupric oxide
CAS NO.:1317-38-0
Empirical Formula: CuO
Molecular Weight: 79.55
MDL number: MFCD00010979
EINECS: 215-269-1
| Pack Size | Price | Stock | Quantity |
| 100g | RMB78.40 | In Stock |
|
| 500g | RMB362.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 1326 °C |
||||||||||||||
| Density | 6.315 |
||||||||||||||
| bulk density | 500kg/m3 |
||||||||||||||
| refractive index | 2.63 |
||||||||||||||
| storage temp. | no restrictions. |
||||||||||||||
| solubility | Aqueous Acid (Slightly), Methanol (Slightly) |
||||||||||||||
| form | powder |
||||||||||||||
| color | Brown to black |
||||||||||||||
| Specific Gravity | 6.3-6.49 |
||||||||||||||
| Odor | at 100.00?%. odorless |
||||||||||||||
| PH | 7 (50g/l, H2O, 20℃)(slurry) |
||||||||||||||
| Water Solubility | insoluble |
||||||||||||||
| Merck | 14,2646 |
||||||||||||||
| crystal system | Monoclinic |
||||||||||||||
| Space group | C2/c |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Dielectric constant | 18.1(Ambient) |
||||||||||||||
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.1 mg/m3; TWA 1 mg/m3 |
||||||||||||||
| Stability: | Stable. Incompatible with reducing agents, hydrogen sulfide, aluminium, alkali metals, finely powdered metals. |
||||||||||||||
| CAS DataBase Reference | 1317-38-0(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Copper(ii) oxide(1317-38-0) |
||||||||||||||
| EPA Substance Registry System | Cupric oxide (1317-38-0) |
Description and Uses
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.Mainly used in wood preservatives, ceramics, and mineral supplements for animal feed.
Copper(II) oxide nanoparticles (NPCuO) have industrial applications as antimicrobial agents in textiles and paints, and catalysts in organic synthesis. They may also be produced from electronic wastes. Cupric oxide poses potential health and environmental concern due to toxic and mutagenic particles generating reactive oxygen species.
Cupric Oxide can be used as a dietary ingredient and as a nutrient. Copper aids in the absorption of iron, in the formation of red blood cells and the proper bone formation and maintenance.
As pigment in glass, ceramics, enamels, porcelain glazes, artificial gems; in manufacture of rayon, other Cu Compounds; in sweetening petroleum gases; in galvanic electrodes; as flux in metallurgy; in correcting Cu deficiencies in soil; as optical-glass polishing agent; in antifouling paints, pyrotechnic compositions; as exciter in phosphor mixtures; as catalyst for organic reactions; in high tempereture superconductors.
Safety
| Symbol(GHS) |  GHS09 |
| Signal word | Warning |
| Hazard statements | H410 |
| Precautionary statements | P273-P391-P501 |
| Hazard Codes | Xn,Xi,N |
| Risk Statements | 22-37-50 |
| Safety Statements | 22-36-61-60-29 |
| RIDADR | UN 3077 9 / PGIII |
| OEB | D |
| OEL | TWA: 0.1 mg/m3 |
| WGK Germany | 1 |
| RTECS | GL7900000 |
| TSCA | Yes |
| HazardClass | 9 |
| HS Code | 28255000 |
| Hazardous Substances Data | 1317-38-0(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 470 mg/kg |
| IDLA | 100 mg Cu/m3 |