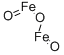Ferric oxide , 10nm, 98%metalsbasis, γ type , 1309-37-1
Synonym(s):
Magnetite;Superparamagnetic iron oxide nanoparticles;Fe NP Biotin;Fe NP COOH;Fe NP NH2
CAS NO.:1309-37-1
Empirical Formula: Fe2O3
Molecular Weight: 159.69
MDL number: MFCD00748135
EINECS: 215-168-2
| Pack Size | Price | Stock | Quantity |
| 25G | RMB55.20 | In Stock |
|
| 100G | RMB159.20 | In Stock |
|
| 500G | RMB559.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 1538°C |
||||||||||||||
| Density | 5.24 |
||||||||||||||
| Flash point: | >230 °F |
||||||||||||||
| storage temp. | 2-8°C |
||||||||||||||
| solubility | It is soluble In Warm Hydrochloric Acid, Slightly Soluble in Sulfuric Acid. |
||||||||||||||
| form | pieces |
||||||||||||||
| color | black |
||||||||||||||
| Specific Gravity | 5.1~5.2 |
||||||||||||||
| PH | 3.7±0.3 |
||||||||||||||
| Water Solubility | INSOLUBLE |
||||||||||||||
| Crystal Structure | Corundum type |
||||||||||||||
| crystal system | Three sides |
||||||||||||||
| Merck | 14,4028 |
||||||||||||||
| Space group | R3c |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 5 mg/m3 OSHA: TWA 10 mg/m3; TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: IDLH 2500 mg/m3; TWA 5 mg/m3 |
||||||||||||||
| Stability: | Stable. |
||||||||||||||
| CAS DataBase Reference | 1309-37-1(CAS DataBase Reference) |
||||||||||||||
| IARC | 3 (Vol. 1, Sup 7) 1987 |
||||||||||||||
| NIST Chemistry Reference | Iron(iii) oxide(1309-37-1) |
||||||||||||||
| EPA Substance Registry System | Ferric oxide (1309-37-1) |
Description and Uses
Iron oxides are produced synthetically and consist essentially of anhydrous and/or hydrated iron oxides. The range of hues includesyellows, reds, browns and blacks. Food quality iron oxides are primarily distinguished from technical grades by the comparatively low levels of contamination by other metals. This is achieved by the selection and control of the source of iron and/or by the extent of chemical purification during the manufacturing process. Iron oxides have been used to color confectionery, fillings and decorations for pastry products, cheese products, fish paste, pet foods, cosmetics and pharmaceutical products.
Red iron oxide (Fe2O3) is an inorganic pigment of either natural or synthetic origin. It is a low chroma red with excellent durability and low cost. Synthetic pigment is made by heating iron sulfate with quicklime in a furnace. The second preparatory technique involves calcining iron sulfate in the presence of air at high temperatures. Natural and oxides of iron are mined either as the mineral hematite (Fe2O3) or as hematite in its hydrated form.
Safety
| Symbol(GHS) |   GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H225-H318 |
| Precautionary statements | P210-P280-P305+P351+P338+P310 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26 |
| OEB | B |
| OEL | TWA: 5 mg/m3 |
| WGK Germany | - |
| RTECS | NO7400000 |
| TSCA | Yes |
| HS Code | 28211000 |
| Hazardous Substances Data | 1309-37-1(Hazardous Substances Data) |
| IDLA | 2,500 mg Fe/m3 |