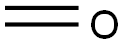Formaldehyde solution , analyticalstandard,100mg/L,in5%ethanol , 50-00-0
Synonym(s):
Formaldehyde Solution;Formaldehydi solutio 35 per centum;Formalin;Formalin, Formaldehyde;Formaline solution, Methanal solution, Methylaldehyde solution
CAS NO.:50-00-0
Empirical Formula: CH2O
Molecular Weight: 30.03
MDL number: MFCD00003274
EINECS: 200-001-8
PRODUCT Properties
| Melting point: | -15°C |
| Boiling point: | 97°C(37% solution),-19.5°C(pure) |
| Density | 1.09 g/mL at 25 °C(lit.) |
| vapor density | 1.03 (vs air) |
| vapor pressure | 52 mm Hg ( 37 °C) |
| refractive index | n |
| Flash point: | 133 °F |
| storage temp. | room temp |
| solubility | water: soluble |
| form | Solution |
| pka | 13.27(at 25℃) |
| color | APHA: ≤10 |
| PH | pH (25℃) : 7.0~7.5 |
| Odor | Pungent odor detectable at 1 ppm |
| Odor Threshold | 0.5ppm |
| explosive limit | 7-73% (v/v) Formaldehyde) |
| Water Solubility | soluble |
| λmax | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,4235 |
| BRN | 1209228 |
| Henry's Law Constant | 2.08(x 10-7 atm?m3/mol) at 25 °C (flow-type generation system, spectrophometry, Kanda et al., 2005) |
| Exposure limits | NIOSH REL: TWA 0.016 ppm, 15-min C 0.1 ppm, IDLH 20 ppm; OSHA PEL: TWA 0.75 ppm, STEL 2 ppm. |
| Dielectric constant | 23.0(Ambient) |
| InChIKey | WSFSSNUMVMOOMR-UHFFFAOYSA-N |
| LogP | 0.350 |
| CAS DataBase Reference | 50-00-0(CAS DataBase Reference) |
| IARC | 1 (Vol. Sup 7, 62, 88, 100F) 2012 |
| NIST Chemistry Reference | Formaldehyde(50-00-0) |
| EPA Substance Registry System | Formaldehyde (50-00-0) |
Description and Uses
Formaldehyde is a colorless, flammable gas with a distinctive pungent odor. It is the simplest aldehyde, which is a class of organic compounds with the carbonyl group bonded to at least one hydrogen atom. Formaldehyde was described by August Wilhelm von Hoff mann (1818–1892) in 1867 after the Russian Aleksandr Butlerov (1828–1886) had inadvertently synthesized it in 1857. Formaldehyde readily dissolves in water to produce a solution called formalin, which is commonly marketed as a 37% solution.
Formaldehyde is used in the manufactureof phenolic resins, cellulose esters, artificialsilk, dyes, explosives, and organic chemicals.Other uses are as a germicide, fungicide, anddisinfectant; in tanning, adhesives, waterproofingfabrics, and for tonic and chromeprinting in photography; and for treating skindiseases in animals. In vitro neutralizationof scorpion venom toxicity by formaldehydehas been reported (Venkateswarlu et al.1988).
Formaldehyde constitutes about 50% ofall aldehydes present in the air. It is oneof the toxic effluent gases emitted fromburning wood and synthetic polymeric substancessuch as polyethylene, nylon 6, andpolyurethane foams. Firefighters have a greaterrisk to its exposure. Incapacitation fromthe toxic effluent gases is reported to occurmore rapidly from the combustion of syntheticpolymers than from that of naturalcellulose materials.
Formaldehyde is directly emitted into theair from vehicles. It is released in traceamounts from pressed wood products suchas particleboard and plywood paneling, fromold “sick” buildings, and from cotton andcotton–polyester fabrics with selected crosslinkfinishes. Formation of formaldehyde hasbeen observed in some frozen gadoid fishdue to enzymic decomposition of the additivetrimethylamine oxide (Rehbein 1985).Its concentration can build up during frozenstorage of fish (Leblanc and Leblanc 1988;Reece 1985). It occurs in the upper atmosphere,cloud, and fog; it also forms inphotochemical smog processes.
Safety
| Symbol(GHS) |    GHS05,GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301+H311-H314-H317-H330-H335-H341-H350-H370 |
| Precautionary statements | P202-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | T |
| Risk Statements | 23/24/25-34-40-43-39/23/24/25-68-45-68/20/21/22 |
| Safety Statements | 36/37-51-45-36/37/39-26-53 |
| OEB | E |
| OEL | TWA: 0.016 ppm, Ceiling: 0.1 ppm [15-minute] |
| RIDADR | UN 1198 3/PG 3 |
| WGK Germany | 2 |
| RTECS | LP8925000 |
| F | 10 |
| Autoignition Temperature | 424 °C for formalin containing 15% methanol |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29121100 |
| Hazardous Substances Data | 50-00-0(Hazardous Substances Data) |
| Toxicity | LD50 oral (rat) 500 mg/kg LD50 skin (rabbit) 270 mg/kg LC50 inhal (rat) 203 mg/m3 (2 h) PEL (OSHA) 1 ppm (1.5 mg/m3) TLV-TWA (ACGIH) 0.3 ppm (ceiling)(0.37 mg/m3) STEL (OSHA) 2 ppm (2.5 mg/m3) |
| IDLA | 20 ppm |