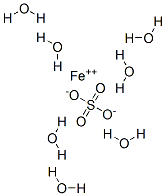
Iron sulfate heptahydrate , 99.95%metalsbasis , 7782-63-0
Synonym(s):
o-Phenanthroline ferrous sulfate complex;1,10-Phenanthroline iron(II) sulfate complex;Ferrous sulfate heptahydrate;Iron vitriol;Iron(II) sulfate heptahydrate
CAS NO.:7782-63-0
Empirical Formula: FeH14O11S
Molecular Weight: 278.01
MDL number: MFCD00036428
EINECS: 616-510-7
| Pack Size | Price | Stock | Quantity |
| 25G | RMB58.40 | In Stock |
|
| 100G | RMB195.20 | In Stock |
|
| 500G | RMB651.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 64 °C |
| Density | 1.898 g/mL at 25 °C (lit.) |
| bulk density | 600kg/m3 |
| vapor pressure | 14.6 mm Hg ( 25 °C) |
| storage temp. | Store at +15°C to +25°C. |
| solubility | 25.6 g/100 mL (20°C) |
| form | Solution |
| color | Slightly greenish to blue |
| Specific Gravity | 1.898 |
| PH | 3.0-4.0 (25℃, 50mg/mL in H2O) |
| Odor | pale bluish-grn. cryst. or gran., odorless |
| Water Solubility | 25.6 g/100 mL (20 ºC) |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,4057 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Stability: | Stable. Substances to be avoided include strong oxidizing agents. Air and moisture sensitive. |
| InChIKey | SURQXAFEQWPFPV-UHFFFAOYSA-L |
| LogP | -1.031 (est) |
| CAS DataBase Reference | 7782-63-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ferrous sulfate heptahydrate (7782-63-0) |
Description and Uses
§184.1315(a) Ferrous sulfate heptahydrate (iron (II) sulfate heptahydrate, FeS04-7H2O) is prepared by the action of sulfuric acid on iron. It occurs as pale, blueish-green crystals or granules. Progressive heating of ferrous sulfate heptahydrate produces ferrous sulfate (dried). Ferrous sulfate (dried) consists primarily of ferrous sulfate monohydrate (CAS No. 17375-41-6) with varying amounts of ferrous sulfate tetrahydrate (CAS No. 20908-72-9) and occurs as a grayish-white to buff-colored powder.
Iron(II) sulfate heptahydrate is used as a precursor to prepare other iron compounds such as a lawn conditioner and a mordant for wool dyeing. It is actively used in the manufacture of ink including iron gall ink. As a reducing agent, it participates in the reduction of chromate in cement. It is also used in industrial water treatment plants to remove phosphate. It is also used in the gold refining process to precipitate metallic gold. Woodworkers use aqueous solutions of ferrous sulfate to color maple wood a silvery hue. It finds application in the treatment of iron chlorosis, which arises due to deficiency of iron in horticulture.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319 |
| Precautionary statements | P264-P270-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 22-36/38 |
| Safety Statements | 36/37/39-46 |
| WGK Germany | 1 |
| RTECS | NO8510000 |
| F | 10-23 |
| TSCA | Yes |
| HS Code | 28332950 |
| Toxicity | LD50 in mice: 65 mg/kg i.v.; 1.52 g/kg orally (Hoppe) |