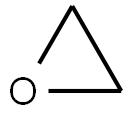
Ethylene oxide , 0.2 mg/ml in isophane , 75-21-8
Synonym(s):
Ethylene oxide solution;Oxirane
CAS NO.:75-21-8
Empirical Formula: C2H4O
Molecular Weight: 44.05
MDL number: MFCD00014482
EINECS: 200-849-9
PRODUCT Properties
| Melting point: | −111 °C(lit.) |
| Boiling point: | 10.7 °C(lit.) |
| Density | 0.882 g/mL at 25 °C(lit.) |
| vapor pressure | 1095 mmHg at 20 °C |
| refractive index | n |
| Flash point: | <-17.7℃ |
| storage temp. | 2-8°C |
| form | Colorless gas |
| Odor | Sweet odor detectable at 257 to 690 ppm |
| Merck | 3802 |
| BRN | 102378 |
| Exposure limits | TLV-TWA 1.8 mg/m3
(1 ppm) (ACGIH),
0.18 mg/m3
(0.1 ppm), 5 ppm/10 min
(NIOSH). |
| Dielectric constant | 14.0(-4℃) |
| InChIKey | IAYPIBMASNFSPL-UHFFFAOYSA-N |
| LogP | -0.30 |
| CAS DataBase Reference | 75-21-8(CAS DataBase Reference) |
| IARC | 1 (Vol. Sup 7, 60, 97, 100F) 2012 |
| EPA Substance Registry System | Ethylene oxide (75-21-8) |
Description and Uses
Ethylene oxide (C2H4O) is a kind of cyclic ether with important industrial applications. Although it is highly toxic and dangerous for household application and consumers to use, it can be used for the manufacture of many important industrial and commercialized products as well as some chemicals and intermediates. For example, it is very useful in the production of detergents, thickeners, solvents, plastics, and many kinds of organic chemicals such as ethylene glycol, ethanolamines, simple and complex glycols, polyglycol ethers, and other compounds. It is also a commonly sterilization methods used in the healthcare industry. In addition, it can be used as an accelerator of maturation of tobacco leaves and fungicide, as well as the main component of thermobaric weapons (fuel-air explosives). In industry, it is generally manufactured through direct oxidation of ethylene. In low doses, it can be used as a pesticide and a sterilizing agent owing to its effect of causing DNA damage. However, this property also make it a potential carcinogen.
ethylene oxide structure
Fumigant for foodstuffs and textiles; to sterilize surgical instruments; agricultural fungicide. In organic syntheses, especially in the production of ethylene glycol. Starting material for the manufacture of acrylonitrile and nonionic surfactants.
Safety
| Symbol(GHS) |      GHS02,GHS04,GHS05,GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H220-H230-H280-H301+H331-H314-H335-H336-H340-H350-H360FD-H372 |
| Precautionary statements | P202-P210-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | F+,T,F |
| Risk Statements | 45-46-12-23-36/37/38-39/23/24/25-23/24/25-11-67-20-36/37-19-6 |
| Safety Statements | 53-45-36/37-16-24/25-23-26 |
| OEB | C |
| OEL | TWA: <0.1 ppm (0.18 mg/m3), Ceiling: 5 ppm (9 mg/m3) [10-min/day] |
| RIDADR | UN 2037 2.3 |
| WGK Germany | 2 |
| RTECS | KX2450000 |
| F | 4.5-31 |
| Autoignition Temperature | 429 °C |
| DOT Classification | 2.3, Hazard Zone D (Gas poisonous by inhalation) |
| HazardClass | 2.3 |
| HS Code | 29101000 |
| Hazardous Substances Data | 75-21-8(Hazardous Substances Data) |
| Toxicity | LD50 oral (rat) 72 mg/kg LC50 inhal (rat) 800 ppm (1600 mg/m3) PEL (OSHA) 1 ppm (2 mg/m3) TLV-TWA (ACGIH) 1 ppm (2 mg/m3) |
| IDLA | 800 ppm |