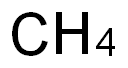Formate , Analysis standard , 64-18-6
Synonym(s):
Formic acid;Methanoic acid solution, Formylic acid solution;Methanoic acid, Formylic acid;Methanoic Acid,Aminic Acid;Methanoic Acid,Aminic Acid, Aminic Acid
CAS NO.:64-18-6
Empirical Formula: CH2O2
Molecular Weight: 46.03
MDL number: MFCD00003274
EINECS: 200-579-1
PRODUCT Properties
| Melting point: | 8.2-8.4 °C (lit.) |
| Boiling point: | 100-101 °C (lit.) |
| Density | 1.22 g/mL at 25 °C (lit.) |
| vapor density | 1.03 (vs air) |
| vapor pressure | 52 mm Hg ( 37 °C) |
| refractive index | n |
| FEMA | 2487 | FORMIC ACID |
| Flash point: | 133 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble1g/10 mL, clear, colorless |
| pka | 3.75(at 20℃) |
| form | Liquid |
| Specific Gravity | 1.216 (20℃/20℃) |
| color | APHA: ≤15 |
| PH | 3.47(1 mM solution);2.91(10 mM solution);2.38(100 mM solution); |
| Odor | at 0.10 % in water. pungent vinegar formyl |
| Odor Type | acetic |
| explosive limit | 12-38%(V) |
| biological source | synthetic |
| Water Solubility | MISCIBLE |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.01 |
| Merck | 14,4241 |
| JECFA Number | 79 |
| BRN | 1209246 |
| Henry's Law Constant | At 25 °C: 95.2, 75.1, 39.3, 10.7, and 3.17 at pH values of 1.35, 3.09, 4.05, 4.99, and 6.21,
respectively (Hakuta et al., 1977) |
| Dielectric constant | 58.0(16℃) |
| Exposure limits | TLV-TWA 5 ppm (~9 mg/m3) (ACGIH,
MSHA, OSHA, and NIOSH); IDLH 100
ppm (180 mg/m3) (NIOSH). |
| Stability: | Stable. Substances to be avoided include strong bases, strong oxidizing agents and powdered metals, furfuryl alcohol. Combustible. Hygroscopic. Pressure may build up in tightly closed bottles, so bottles should be opened carefully and vented periodically. |
| InChIKey | BDAGIHXWWSANSR-UHFFFAOYSA-N |
| LogP | -0.540 |
| CAS DataBase Reference | 64-18-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Formic acid(64-18-6) |
| EPA Substance Registry System | Formic acid (64-18-6) |
Description and Uses
Formic acid is a clear, colorless liquid with a pungent odor.
Formic acid was first isolated from certain ants and was named
after the Latin formica, meaning ant. It is made by the action of
sulfuric acid on sodium formate, which is produced from
carbon monoxide and sodium hydroxide. It is also produced as
a by-product in the manufacture of other chemicals such as
acetic acid.
It can be anticipated that use of formic acid will continuously
increase as it replaces inorganic acids and has a potential
role in new energy technology. Formic acid toxicity is of
a special interest as the acid is the toxic metabolite of methanol.
Formic acid has a number of commercial uses. It is used in the leather industry to degreaseand remove hair from hides and as an ingredient in tanning formulations. It is used as alatex coagulant in natural rubber production. Formic acid and its formulations are used aspreservatives of silage. It is especially valued in Europe where laws require the use of naturalantibacterial agents rather than synthetic antibiotics. Silage is fermented grass and crops thatare stored in silos and used for winter feed. Silage is produced during anaerobic fermentationwhen bacteria produce acids that lower the pH, preventing further bacterial action. Acetic acidand lactic acid are the desired acids during silage fermentation. Formic acid is used in silageprocessing to reduce undesirable bacteria and mold growth. Formic acid reduces Clostridiabacteria that would produce butyric acid causing spoilage. In addition to preventing silagespoilage, formic acid helps preserve protein content, improves compaction, and preservessugar content. Formic acid is used as a miticide by beekeepers.
Safety
| Symbol(GHS) |   GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H302-H314-H331 |
| Precautionary statements | P261-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | T,C,Xi |
| Risk Statements | 23/24/25-34-40-43-35-36/38-10 |
| Safety Statements | 36/37-45-26-23-36/37/39 |
| RIDADR | UN 1198 3/PG 3 |
| OEB | B |
| OEL | TWA: 5 ppm (9 mg/m3) |
| WGK Germany | 2 |
| RTECS | LP8925000 |
| F | 10 |
| Autoignition Temperature | 1004 °F |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 29151100 |
| Hazardous Substances Data | 64-18-6(Hazardous Substances Data) |
| Toxicity | LD50 in mice (mg/kg): 1100 orally; 145 i.v. (Malorny) |
| IDLA | 30 ppm |