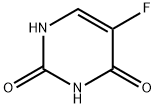
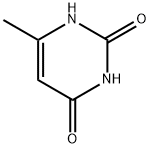
5-Fluorouracil , 99% , 51-21-8
Synonym(s):
5-FU;5-Fluorouracil;5-Fluorouracil - CAS 51-21-8 - Calbiochem;2,4-Dihydroxy-5-fluoropyrimidine;5-Fluoro-2,4(1H,3H)-pyrimidinedione
CAS NO.:51-21-8
Empirical Formula: C4H3FN2O2
Molecular Weight: 130.08
MDL number: MFCD00006018
EINECS: 200-085-6
| Pack Size | Price | Stock | Quantity |
| 1G | RMB24.00 | In Stock |
|
| 5G | RMB36.80 | In Stock |
|
| 25G | RMB72.80 | In Stock |
|
| 100G | RMB149.60 | In Stock |
|
| 500G | RMB663.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 282-286 °C (dec.) (lit.) |
| Boiling point: | 190-200°C/0.1mmHg |
| Density | 1.4593 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 10 mg/mL, clear |
| form | powder |
| pka | pKa 8.0±0.1 (H2O) (Uncertain);3.0±0.1(H2O) (Uncertain) |
| color | white |
| PH | 4.3-5.3 (10g/l, H2O, 20℃) |
| Water Solubility | 12.2 g/L 20 ºC |
| Sensitive | Air Sensitive |
| Merck | 14,4181 |
| BRN | 127172 |
| Stability: | Stable. Light sensitive. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| InChIKey | GHASVSINZRGABV-UHFFFAOYSA-N |
| CAS DataBase Reference | 51-21-8(CAS DataBase Reference) |
| IARC | 3 (Vol. 26, Sup 7) 1987 |
| NIST Chemistry Reference | 2,4-Pyrimidinedione, 5-fluoro-(51-21-8) |
| EPA Substance Registry System | 5-Fluorouracil (51-21-8) |
Description and Uses
5-Fluorouracil (5-FU) is a prodrug form of the thymidylate synthase inhibitor fluorodeoxyuridylate (FdUMP). It is also converted to the active metabolites FUTP and FdUTP, which induce RNA and DNA damage, respectively. In vivo, 5-FU (15 mg/kg) when administered in combination with docetaxel reduces tumor growth in B88 and CAL 27 oral squamous cell carcinoma (OSCC) mouse xenograft models. Formulations containing 5-FU have been used in the treatment of colorectal, breast, gastric, and pancreatic cancers.
5-Fluorouracil is used as an antitumor agent in the treatment of anal, breast, colorectal, oesophageal, stomach, pancreatic and skin cancers. It finds application as a suicide inhibitor due to its irreversible inhibition of thymidylate synthase. It is also used in the treatment of actinic keratoses and bowen's disease. Further, it serves as a potent antineoplastic agent in clinical use. In addition to this, it acts as a DNA synthesis inhibitor.
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301-H351 |
| Precautionary statements | P201-P202-P264-P270-P280-P301+P310 |
| Hazard Codes | Xn,T,C,Xi |
| Risk Statements | 22-20/21/22-52-25 |
| Safety Statements | 36-36/37-36/37/39-22-45-26 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | YR0350000 |
| F | 10-23 |
| Hazard Note | Irritant/Highly Toxic |
| TSCA | T |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29335995 |
| Hazardous Substances Data | 51-21-8(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 230 mg/kg |