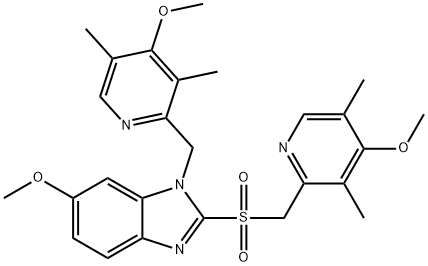
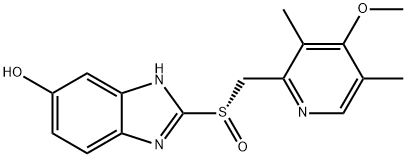
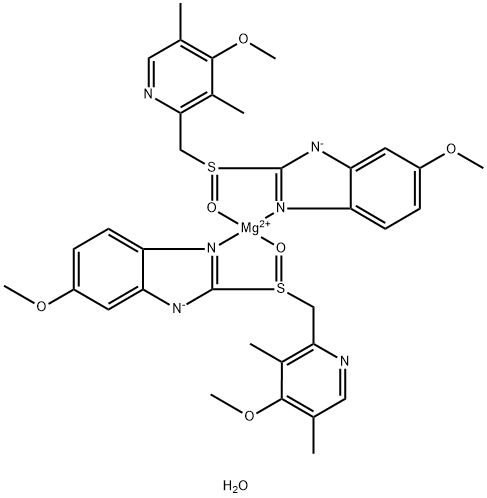
A4037912
Esomeprazole magnesium trihydrate , Pharmaceutical level , 217087-09-7
Synonym(s):
(S)-Omeprazole magnesium trihydrate
CAS NO.:217087-09-7
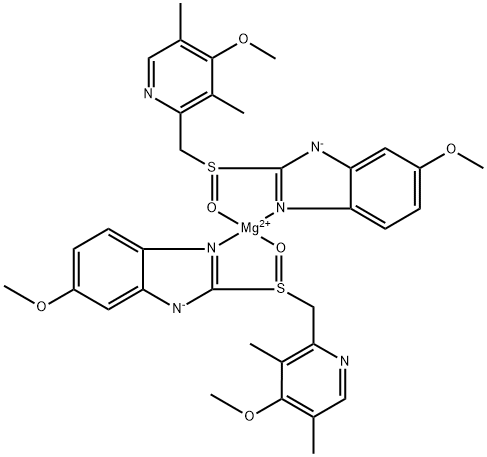
Empirical Formula: C34H38MgN6O7S2
Molecular Weight: 731.14
MDL number: MFCD07698573
EINECS: 669-839-3
| Pack Size | Price | Stock | Quantity |
| 1G | RMB23.20 | In Stock |
|
| 5g | RMB67.20 | In Stock |
|
| 10G | RMB111.20 | In Stock |
|
| 25g | RMB218.40 | In Stock |
|
| 100g | RMB788.00 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 184-189°C (dec.) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Slightly soluble in water, soluble in methanol, practically insoluble in heptane. |
| form | Solid |
| color | White to off-white |
| InChI | InChI=1S/2C17H18N3O3S.Mg.H2O/c2*1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17;;/h2*5-8H,9H2,1-4H3;;1H2/q2*-1;+2; |
| InChIKey | VEVZQDGATGBLIC-UHFFFAOYSA-N |
| SMILES | C(C1N=CC(C)=C(OC)C=1C)S1C2[N-]C3=CC(OC)=CC=C3N=2[Mg+2]2(O=S(CC3N=CC(C)=C(OC)C=3C)C3[N-]C4=CC(OC)=CC=C4N2=3)O=1.O |
| CAS DataBase Reference | 217087-09-7(CAS DataBase Reference) |
Description and Uses
esomeprazole magnesium trihydrate for the healing of peptic ulcers associated with non-steroidal anti-inflammatory drug NSAID (non-selective and COX-2 selective) therapy.
- Zollinger-Ellison syndrome
- Peptic ulcer, site unspecified
- Duodenal ulcer
- Gastric ulcer
- Gastro-oesophageal reflux disease
- Gastro-Oesophageal Reflux Disease (GORD):
- treatment of erosive reflux oesophagitis;
- long-term management of patients with healed oesophagitis to prevent relapse;
- symptomatic treatment of gastro-oesophageal reflux disease (GORD).
- healing of duodenal ulcer associated with Helicobacter pylori;
- eradication of Helicobacter pylori in patients with active or healed peptic ulcer.
- Short-term treatment of upper gastrointestinal symptoms associated with non-steroidal anti-inflammatory drug (NSAID) (non-selective and COX-2 selective) therapy.
- Healing of gastric ulcers associated with non-steroidal anti-inflammatory drug NSAID (non-selective and COX-2 selective) therapy.
- Prevention of gastric and duodenal ulcers associated with non-steroidal anti-inflammatory drug NSAID (non-selective and COX-2 selective) therapy in patients at risk.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P301+P312+P330 |
| Hazard Codes | Xn |
| Risk Statements | 22 |
| WGK Germany | 3 |
| HS Code | 2933399090 |

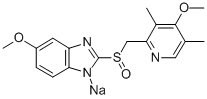
![2-[[(4-Methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole](https://img.chemicalbook.com/CAS/20150408/GIF/73590-60-0.gif)