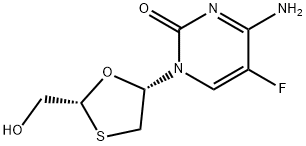
Emtricitabine , 98% , 143491-57-0
Synonym(s):
(−)-2′3′-Dideoxy-5-fluoro-3′-thiacytidine;(2R,5S)-4-Amino-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one;FTC
CAS NO.:143491-57-0
Empirical Formula: C8H10FN3O3S
Molecular Weight: 247.25
MDL number: MFCD00870151
EINECS: 604-363-1
| Pack Size | Price | Stock | Quantity |
| 25MG | RMB23.20 | In Stock |
|
| 50MG | RMB23.20 | In Stock |
|
| 250MG | RMB24.00 | In Stock |
|
| 1g | RMB40.80 | In Stock |
|
| 5g | RMB137.60 | In Stock |
|
| 25G | RMB639.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 136-140°C |
| alpha | D25 -133.60° (c = 0.23 in MeOH) |
| Boiling point: | 443.3±55.0 °C(Predicted) |
| Density | 1.82±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly) |
| form | Solid |
| pka | 13.83±0.10(Predicted) |
| color | White to Pale Yellow |
| optical activity | [α]/D -150 to -125°, c =0.25 in methanol |
| λmax | 280nm(H2O)(lit.) |
| Merck | 14,3565 |
| BCS Class | 1 |
| InChIKey | XQSPYNMVSIKCOC-NTSWFWBYSA-N |
| CAS DataBase Reference | 143491-57-0(CAS DataBase Reference) |
Description and Uses
Emtricitabine is a synthetic nucleoside inhibitor of HIV-1 reverse transcriptase. Its mechanism of action entails the phosphorylation of the oxathiolane carbinol function by cellular enzymes to form the corresponding 5′-triphosphate, which competes with the endogenous 2′-deoxycytidine 5′-triphosphate substrate. The in vitro activity of emtricitabine ranges from IC50 of 0.00013 to 0.64 mM against lymphoblastoid cell lines, MAGI-CCR5 cell lines, and peripheral blood mononuclear cells. Emtricitabine is prepared in about 16 steps from L-gulose, from which the O–C–S carbon stereochemistry is derived in the formation of substituted key 4-O-Acetyl-1,3- oxathiolane intermediate. This intermediate is coupled with N-benzoyl-O-trimethylsilyl- 5-fluoro-cytosine in the presence of trimethylsilyl triflate. The resulting anomeric mixture is separated by silica chromatography and subjected to two deprotection steps. Emtricitabine resistant isolates (M184V/I) were cross-resistant to lamivudine (desfluoro version of emtricitabine) and zalcitabine but remained sensitive to abacavir, didanosine, stavudine, tenofovir, zidovudine, and non-nucleoside reverse transcriptase inhibitors (delavirdine, efavirenz, and nevirapine). In a 48-week clinical trial using emtricitabine with didanosine and efavirin versus stavudine, didanosine and efavirenz produced 81 versus 68% responder rate respectively. In one study, 37.5% of treatment na?¨ve patients that were not achieving successful viral levels were found to exhibit a reduced potency toward emtricitabine. This resistance was due to M184V/I mutation in the HIV reverse transcriptase gene. Recommended dosing of emtricitabine is 200 mg (capsule) once per day. It is 93% bio-available and has a plasma half-life about 10 h, with 86% renal clearance. Side effects associated with emtricitabine treatment were generally mild to moderate and included headache, diarrhea, nausea, and rash. A generally mild and asymptomatic hyperpigmentation of the palms and/or soles was also observed.
anti-Alzheimer’s treatment
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| RTECS | UW7360500 |
| HS Code | 2934990002 |
| Hazardous Substances Data | 143491-57-0(Hazardous Substances Data) |

![(2S)-2-[[[(1,1-Dimethylethyl)diphenylsilyl]oxy]methyl]-1,3-oxathiolan-5-ol 5-Acetate](https://img.chemicalbook.com/CAS/20180601/GIF/202532-88-5.gif)