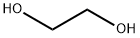Ethylene glycol , StandardforGC,≥99.5% , 107-21-1
Synonym(s):
Ethylene Glycol;1,2-Ethanediol;Monoethylene glycol;Ethylene glycol in dimethyl sulfoxide;Ethylene glycol solution
CAS NO.:107-21-1
Empirical Formula: C2H6O2
Molecular Weight: 62.07
MDL number: MFCD00002885
EINECS: 203-473-3
| Pack Size | Price | Stock | Quantity |
| 5ML | RMB44.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | -13 °C (lit.) |
| Boiling point: | 195-198 °C |
| Density | 1.113 g/mL at 25 °C (lit.) |
| vapor density | 2.1 (vs air) |
| vapor pressure | 0.08 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 230 °F |
| storage temp. | 2-8°C |
| solubility | water: miscible |
| form | Viscous Liquid |
| pka | 14.22(at 25℃) |
| color | blue |
| Relative polarity | 0.79 |
| PH | 6-7.5 (100g/l, H2O, 20℃) |
| Odor | Odorless |
| explosive limit | 3.2%(V) |
| Water Solubility | miscible |
| FreezingPoint | -11.5℃ |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: ≤0.03 λ: 280 nm Amax: ≤0.01 |
| Merck | 14,3798 |
| BRN | 505945 |
| Exposure limits | Ceiling limit in air for vapor and mist
50 ppm (~125 mg/m3) (ACGIH); TWA 10
mg/m3 (particulates) (MSHA). |
| Dielectric constant | 37.0(20℃) |
| LogP | -1.36 at 25℃ |
| Surface tension | 47.7mN/m at 20°C |
| CAS DataBase Reference | 107-21-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2-Ethanediol(107-21-1) |
| EPA Substance Registry System | Ethylene glycol (107-21-1) |
Description and Uses
Ethylene glycol was first synthesized in 1859; however, it did not become a public health concern until after World War II. In fact, the first published series of deaths from ethylene glycol consumption involved 18 soldiers who drank antifreeze as a substitute for ethanol. Despite the early recognition that patients who drank ethanol in addition to ethylene glycol had prolonged survival when compared to those drinking ethylene glycol alone, antidotal treatment of ethylene glycol toxicity with ethanol was not evaluated until the 1960s. Today, ethylene glycol poisoning continues to be a public health problem, particularly in the southeastern United States. In 2009, US poison control centers received 5282 calls about possible ethylene glycol exposures, and the toxicology community believes these exposures are underreported.
Antifreeze in cooling and heating systems. In hydraulic brake fluids and de-icing solutions. Industrial humectant. Ingredient of electrolytic condensers (where it serves as solvent for boric acid and borates). Solvent in the paint and plastics industries. In the formulation of printers' inks, stamp pad inks, ball-point pen ink. Softening agent for cellophane. Stabilizer for soybean foam used to extinguish oil and gasoline fires. In the synthesis of safety explosives, glyoxal, unsatd ester type alkyd resins, plasticizers, elastomers, synthetic fibers (Terylene, Dacron), and synthetic waxes. To create artificial smoke and mist for theatrical uses.
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302-H373 |
| Precautionary statements | P260-P264-P270-P301+P312-P314-P501 |
| Hazard Codes | Xn |
| Risk Statements | 22-36-41 |
| Safety Statements | 26-39-36/37/39 |
| WGK Germany | 3 |
| RTECS | KW2975000 |
| Autoignition Temperature | 752 °F |
| TSCA | Yes |
| HS Code | 29053100 |
| Hazardous Substances Data | 107-21-1(Hazardous Substances Data) |
| Toxicity | LD50 in rats, guinea pigs (g/kg): 8.54, 6.61 orally (Smyth); in mice (ml/kg): 13.79 orally (Bornmann) |