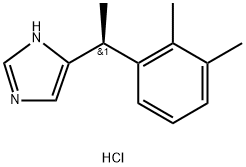
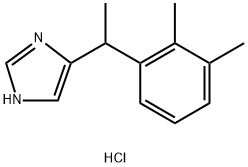
Dexmedetomidine HCl , ≥98% , 145108-58-3
Synonym(s):
(+)-Medetomidine hydrochloride;(S)-Medetomidine hydrochloride;4-[(S)-α,2,3-Trimethylbenzyl]imidazole monohydrochloride;5-[(1S)-1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole hydrochloride;Dexmedetomidine hydrochloride
CAS NO.:145108-58-3
Empirical Formula: C13H17ClN2
Molecular Weight: 236.74
MDL number: MFCD07772269
EINECS: 682-047-2
| Pack Size | Price | Stock | Quantity |
| 5MG | RMB238.40 | In Stock |
|
| 10MG | RMB316.00 | In Stock |
|
| 50MG | RMB886.40 | In Stock |
|
| 100mg | RMB1284.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 156.5-157.5° |
| alpha | +52.4° (c = 1 in water) |
| Density | 1.17 g/cm3 |
| storage temp. | 2-8°C |
| solubility | H2O: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| optical activity | [α]/D +48 to +58°, c = 1 in H2O |
| Water Solubility | H2O: 20mg/mL, clear |
| Merck | 14,2946 |
| Stability: | Hygroscopic |
| InChI | InChI=1/C13H16N2.ClH/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13;/h4-8,11H,1-3H3,(H,14,15);1H/t11-;/s3 |
| InChIKey | VPNGEIHDPSLNMU-MERQFXBCSA-N |
| SMILES | [C@@H](C1NC=NC=1)(C1C=CC=C(C)C=1C)C.Cl |&1:0,r| |
| CAS DataBase Reference | 145108-58-3(CAS DataBase Reference) |
Description and Uses
Dexmedetomidine was launched in the US as an i.v. infusion for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care unit, This imidazole derivative is the (S)-enantiomer of medetomidine that can be obtained via fractional crystallization of the tartrate salt of the racemic mixture. Dexmedetomidine is a full agonist of α2 adrenoceptors with 1300-fold selectivity versus α1 compared to the less selective partial α2 agonist clonidine, a veterinary hypnotic. Dexmedetomidine is unique compared with currently available sedatives in that it provides sedation, analgesia and anxiolysis with the ability of patients to be easily awakened. Besides, it causes minimal respiratory depression unlike other available drugs such as benzodiazepines or opioids. Pharmacological studies in transgenic mice showed that the sedative, anesthetic and analgesic effects of dexmedetomidine are specifically due to the stimulation of the a2A subtype receptor. Like other α2 adrenoceptor agonists, dexmedetomidine can provoke hypotension and bradycardia probably because of its non-selective action on peripheral a2B subtype receptors in vascular smooth muscle. Dexmedetomidine is extensively metabolized into methyl and glucuronide conjugates which are mainly eliminated by renal excretion. It was found to be a CYP2D6 inhibitor but less potent than the clinically relevant standard quinidine.
highly selective, potent α2-adrenoceptor agonist, analgesic, anxiolytic, bradycardic, hypotensive, sedative, hypothermic
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P264-P270-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36/37/39 |
| WGK Germany | 3 |
| RTECS | NI5156750 |
| HS Code | 2933290000 |