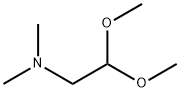
Dimethoxy methane , 98% , 109-87-5
Synonym(s):
Dimethoxymethane;Formal, Methylal, Dimethoxymethane;Formaldehyde dimethyl acetal;Methylal
CAS NO.:109-87-5
Empirical Formula: C3H8O2
Molecular Weight: 76.09
MDL number: MFCD00008495
EINECS: 203-714-2
PRODUCT Properties
| Melting point: | -105 °C |
| Boiling point: | 41-43 °C |
| Density | 0.860 g/mL at 20 °C(lit.) |
| vapor density | 2.6 (vs air) |
| vapor pressure | 6.38 psi ( 20 °C) |
| refractive index | n |
| Flash point: | -18 °C |
| storage temp. | Store at <= 20°C. |
| solubility | 32.3 g/100 mL (16°C) |
| form | Liquid |
| pka | 3.1[at 20 ℃] |
| color | Clear colorless |
| Odor | Mild, ethereal; chloroform-like. |
| PH | pH (12.5vol % , 25℃) : 4.5~6.6 |
| explosive limit | 1.6-17.6%(V) |
| Water Solubility | 32.3 g/100 mL (16 ºC) |
| Sensitive | Moisture Sensitive |
| Merck | 14,6012 |
| BRN | 1697025 |
| Henry's Law Constant | (x 10-4 atm?m3/mol):
1.73 at 25 °C (Hine and Mookerjee, 1975) |
| Exposure limits | NIOSH REL: TWA 1,000 ppm (3,100 mg/m3), IDLH 2,200 ppm; OSHA PEL:
TWA 1,000 ppm; ACGIH TLV: TWA 1,000 ppm (adopted). |
| Dielectric constant | 2.7(20℃) |
| Stability: | Stable. Extremely flammable. Readily forms explosive mixtures with air. Note low flash point and wide explosive limits. Incompatible with strong oxidizing agents, strong acids. May form explosive peroxides upon exposure to air. |
| LogP | 0 |
| CAS DataBase Reference | 109-87-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Methane, dimethoxy-(109-87-5) |
| EPA Substance Registry System | Dimethoxymethane (109-87-5) |
Description and Uses
Methylal is a colorless liquid with a pungent odor. Molecular weight= 76.11; Specific gravity (H2O:1)= 0.86;Boiling point=43.9℃; Freezing/Melting point=105℃; Vapor pressure 330 mmHg at 20℃; Flash point=32℃; Autoignition temperature=237℃. Explosive limits: LEL= 2.2%; UEL= 13.8%. Hazard Identification (based on NFPA-704 M Rating System): Health 2, Flammability 3, Reactivity 2. Soluble in water; solubility= 33%.
Dimethoxymethane (Methylal) is a valuable extraction solvent for pharmaceutical products and a stable, inexpensive solvent for Grignard reactions. Methylal is stable under alkaline and mild acidic conditions. Its dissolving ability is stronger than ether, acetone, and methanol and azeotrope can dissolve nitrocellulose with high nitrogen content. Dimethoxymethane is mainly used in the production of anion-exchange resins, and also as solvents and special fuels.
Safety
| Symbol(GHS) |  GHS02 |
| Signal word | Danger |
| Hazard statements | H225 |
| Precautionary statements | P210-P233-P240-P241-P242-P243 |
| Hazard Codes | F,Xi |
| Risk Statements | 11-19-36-36/37/38 |
| Safety Statements | 16-26-33-7/9-37/39 |
| OEB | A |
| OEL | TWA: 1000 ppm (3100 mg/m3) |
| RIDADR | UN 1234 3/PG 2 |
| WGK Germany | 1 |
| RTECS | PA8750000 |
| F | 21 |
| Autoignition Temperature | 459 °F |
| TSCA | Yes |
| HS Code | 2911 00 00 |
| HazardClass | 3 |
| PackingGroup | II |
| Hazardous Substances Data | 109-87-5(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 6653 mg/kg |
| IDLA | 2,200 ppm [10% LEL] |