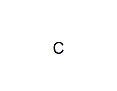Activated charcoal , Cell culture level , 7440-44-0
Synonym(s):
Activated carbon;Carbon;Activated charcoal;rGO;Charcoal activated
CAS NO.:7440-44-0
Empirical Formula: C
Molecular Weight: 12.011
MDL number: MFCD00133992
EINECS: 231-153-3
| Pack Size | Price | Stock | Quantity |
| 500G | RMB342.40 | In Stock |
|
| 2.5KG | RMB1256.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 3550 °C (lit.) |
| Boiling point: | 500-600 °C(lit.) |
| Density | ~1.7 g/mL at 25 °C(lit.) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| Flash point: | >230 °F |
| storage temp. | no restrictions. |
| solubility | Insoluble. |
| form | rod |
| color | Black |
| Specific Gravity | 1.8~2.1 (amorphous) |
| PH | 6-9 |
| Odor | at 100.00?%. odorless |
| Resistivity | 1375 μΩ-cm, 20°C (graphite) |
| Water Solubility | Insoluble in water. |
| Merck | 14,1807 |
| BRN | 4360473 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Combustible. Highly flammable in powdered form. |
| CAS DataBase Reference | 7440-44-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Carbon(7440-44-0) |
| EPA Substance Registry System | Carbon (7440-44-0) |
Description and Uses
All our SWNTs come packed as dry powders, which can be dispersed within the user's solvent of choice.
There are many uses for the very versatile element carbon. It, no doubt, forms morecompounds than any other element, particularly in the world of modern carbon chemistry.Carbon’s nature allows the formation-rings and straight- and branched-chains types of compoundsthat are capable of adding hydrogen as well as many different types of elemental atomsto these structures. (See figure 5 in the book’s section titled “Atomic Structure” for a depictionof a snake eating its tail as an analogy for the carbon ring of benzene.) In addition, theseringed, straight, and branched carbon molecules can be repeated over and over to form verylarge molecules such as the polymers, proteins, and carbohydrates that are required for life.
Carbon is an excellent reducing agent because it readily combines with oxygen to form COand CO2. Thus, in the form of coke in blast furnaces, it purifies metals by removing the oxidesand other impurities from iron.
Carbon, as graphite, has strong electrical conductivity properties. It is an importantcomponent in electrodes used in a variety of devices, including flashlight cells (batteries).Amorphous carbon has some superconduction capabilities.
Graphite is used for the “lead” in pencils, as a dry lubricant, and as electrodes in arc lamps.Of course, carbon is a popular jewelry item (e.g., diamonds).
Future uses of carbon in the forms of fullerenes (C60 up to C240) and applications of nanotechnologywill provide many new and improved products with unusual properties.
Safety
| Symbol(GHS) |  GHS02 |
| Signal word | Danger |
| Hazard statements | H251 |
| Precautionary statements | P235-P280-P403+P235-P407-P420 |
| Hazard Codes | F,Xn,Xi |
| Risk Statements | 36/37-36/37/38-20-10-11 |
| Safety Statements | 26-36-24/25-22-36/37 |
| RIDADR | UN 1325 4.1/PG 3 |
| WGK Germany | 3 |
| RTECS | FF5250100 |
| Autoignition Temperature | 842 °F |
| TSCA | Yes |
| HazardClass | 4.2 |
| PackingGroup | III |
| HS Code | 38021000 |
| Hazardous Substances Data | 7440-44-0(Hazardous Substances Data) |
| Toxicity | LD50 intravenous in mouse: 440mg/kg |