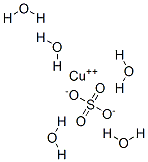
Copper sulfate pentahydrate , Cell culture level, ≥98% , 7758-99-8
Synonym(s):
Copper(II) sulfate pentahydrate;Cupric sulfate pentahydrate;Cupric sulfate standard;Sulfuric acid, copper(II) salt (1:1) pentahydrate;Blue Vitriol
CAS NO.:7758-99-8
Empirical Formula: CuH10O9S
Molecular Weight: 249.68
MDL number: MFCD00149681
EINECS: 616-477-9
| Pack Size | Price | Stock | Quantity |
| 500G | RMB478.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 110 °C (dec.)(lit.) |
| Density | 2.284 |
| vapor pressure | 7.3 mm Hg ( 25 °C) |
| refractive index | 1.537 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 320 g/L (20°C) |
| form | Solid |
| Specific Gravity | 2.284 |
| color | fine blue crystals |
| Odor | blue cryst. or cryst. gran. or powd., odorless |
| PH | 3.5-4.5 (25℃, 50mg/mL in H2O) |
| Water Solubility | 320 g/L (20 ºC) |
| Merck | 14,2653 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| CAS DataBase Reference | 7758-99-8(CAS DataBase Reference) |
| EPA Substance Registry System | Copper sulfate pentahydrate (7758-99-8) |
Description and Uses
Copper(II) sulfate pentahydrate is known as blue vitriol. It is an odorless blue crystal that readily dissolves in water. It is also soluble in methanol, glycerol and slightly soluble in ethanol. The highly toxic, non-combustible has a nauseating metallic taste and turns white when dehydrated. Structurally, in the pentahydrate molecule, each copper(II) ions is surrounded by four water molecules in the corners and the fifth water molecule is attached by hydrogen bonding. Copper (II) sulfate has many applications including preparation of Bordeaux mixture, a fungicide preparation. Electroplating, timber preservation and textile industry use copper (II) sulfate.
Anhydr salt for detecting and removing trace amounts of water from alcohols and other organic Compounds; as fungicide. Pentahydrate as agricultural fungicide, algicide, bactericide, herbicide; food and fertilizer additive; in insecticide mixtures; in manufacture of other Cu salts; as mordant in textile dyeing; in preparation of azo dyes; in preserving hides; in tanning leather; in preserving wood; in electroplating solutions; as battery electrolyte; in laundry and metal-marking inks; in petroleum refining; as flotation agent; pigment in paints, varnishes and other materials; in mordant baths for intensifying photographic negatives; in pyrotechnic compositions; in water-resistant adhesives for wood; in metal coloring and tinting baths; in antirusting compositions for radiator and heating systems; as reagent toner in photography and photoengraving; etc.
Safety
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302-H318-H410 |
| Precautionary statements | P264-P270-P273-P280-P301+P312-P305+P351+P338 |
| Hazard Codes | Xn,N,Xi |
| Risk Statements | 22-36/38-50/53-52/53-36-36/37/38-20/21/22-51/53 |
| Safety Statements | 22-60-61-26-36 |
| RIDADR | UN 3288 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | GL8900000 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 28332500 |
| Hazardous Substances Data | 7758-99-8(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 960 mg/kg (Smyth) |