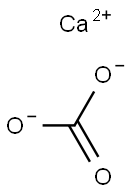
Calcium carbonate , 99.5%,≤30μm , 471-34-1
Synonym(s):
Calcii carbonas;Calcium carbonate;Carbonic Acid Calcium Salt;Carbonic Acid Calcium Salt, Calcium Carbonate
CAS NO.:471-34-1
Empirical Formula: CCaO3
Molecular Weight: 100.087
MDL number: MFCD00010906
EINECS: 207-439-9
| Pack Size | Price | Stock | Quantity |
| 500G | RMB55.20 | In Stock |
|
| 2.5KG | RMB223.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 825 °C |
| Boiling point: | 800 °C |
| Density | 2.93 g/mL at 25 °C (lit.) |
| bulk density | 300-1400kg/m3 |
| refractive index | 1.6583 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 5 M HCl: 0.1 M at 20 °C, clear, colorless |
| form | random crystals |
| Specific Gravity | 2.93 |
| color | White-beige to slightly beige-gray |
| Odor | Odorless |
| PH Range | 8 |
| PH | 9.91(1 mM solution);9.91(10 mM solution);9.91(100 mM solution); |
| Flame Color | Red-orange |
| Water Solubility | Insoluble |
| λmax | λ: 260 nm Amax: ≤0.09 λ: 280 nm Amax: ≤0.06 |
| Merck | 14,1657 |
| BRN | 8008338 |
| Solubility Product Constant (Ksp) | pKsp: 8.54 |
| Exposure limits | NIOSH: TWA 10 mg/m3; TWA 5 mg/m3 |
| Dielectric constant | 6.1(Ambient) |
| Stability: | Stable. Incompatible with acids, fluorine, ammonium salts, alum. |
| InChIKey | VTYYLEPIZMXCLO-UHFFFAOYSA-L |
| CAS DataBase Reference | 471-34-1(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium carbonate (471-34-1) |
Description and Uses
Calcium carbonate occurs in nature as limestone in various forms, such as marble, chalk, and coral. It is probably the most widely-used raw material in the chemical industry. It has numerous applications, primarily to produce cement, mortars, plasters, refractories, and glass as building materials. It also is used to produce quicklime, hydrated lime and a number of calcium compounds. It is produced either as powdered or precipitated calcium carbonate. The latter consists of finer particles of greater purity and more uniform size. They also have many important commercial applications. Various grades of precipitated calcium carbonate are used in several products, such as textiles, papers, paints, plastics, adhesives, sealants, and cosmetics.
calcium carbonate block
Humans primarily use calcium carbonate as a primary source of calcium to combat osteoporosis. Most limestone is used today as construction material. In addition to its use as a construction material, calcium carbonate is also used in numerous industrial processes. Two forms commonly used are ground calcium carbonate (gcc) and precipitated calcium carbonate (pcc).
Calcium carbonate is used widely in papermaking as filler and coating pigment to whiten paper. Calcium carbonate is used in place of more expensive optical brightening agents in paper and as a fill to replace more expensive wood pulp fiber; it also helps control the pH in an alkaline range.
The second most common industrial use of calcium carbonate (after papermaking) representing the largest use of gcc is in the production of plastics. It is used in the production of polyvinyl chloride (PVC), thermoset polyesters, and polyolefins. Calcium carbonate can be used to replace resins that are more expensive. Similar to its use in the paper industry, it is used as an optical brightener and whitening agent. It also is used to increase strength and absorb heat during exothermic processes.
Calcium carbonate is also used in the production of polyethylene and polypropylene. It is an additive to paints and coatings for several purposes including particle size distribution, opacity control, weather resistance, pH control, and anticorrosion. Calcium carbonate is used to buff er acidic soils.
Calcium carbonate has also been used to mitigate the effects of acid precipitation on water bodies. Another environmental application of calcium carbonate is for gas desulfurization in scrubbers used to reduce sulfur emissions from air pollution sources.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P280-P271 |
| Hazard Codes | Xi |
| Risk Statements | 37/38-41-36/38-36 |
| Safety Statements | 26-36/37/39-37/39-37 |
| OEB | B |
| OEL | TWA: 10 mg/m3 (total) |
| WGK Germany | - |
| RTECS | FF9335000 |
| TSCA | Yes |
| HS Code | 28365000 |
| Hazardous Substances Data | 471-34-1(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 6450 mg/kg LD50 dermal Rat > 2000 mg/kg |