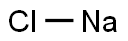Sodium chloride , For cell and insect cell culture, ≥99.5%(T) , 7647-14-5
Synonym(s):
NaCl;Sodium chloride;NaCl 10 Tablets;Natrii chloridum;Physiological Saline, tablets
CAS NO.:7647-14-5
Empirical Formula: NaCl
Molecular Weight: 58.4428
MDL number: MFCD00003477
EINECS: 231-598-3
| Pack Size | Price | Stock | Quantity |
| 500G | RMB148.00 | In Stock |
|
| 2.5KG | RMB309.60 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 801 °C (lit.) |
||||||||||||||
| Boiling point: | 1465 °C/1 atm (lit.) |
||||||||||||||
| Density | 1.199 g/mL at 20 °C |
||||||||||||||
| bulk density | 1140kg/m3 |
||||||||||||||
| vapor pressure | 1 mm Hg ( 865 °C) |
||||||||||||||
| refractive index | n |
||||||||||||||
| Flash point: | 1413°C |
||||||||||||||
| storage temp. | +15C to +30C |
||||||||||||||
| solubility | H2O: soluble |
||||||||||||||
| form | tablets |
||||||||||||||
| Specific Gravity | 2.165 |
||||||||||||||
| color | White |
||||||||||||||
| PH | 5.5-6.5(1 tablet in 100 mL purified water) |
||||||||||||||
| Flame Color | Orange |
||||||||||||||
| Water Solubility | 360 g/L (20 ºC) |
||||||||||||||
| Sensitive | Hygroscopic |
||||||||||||||
| λmax | λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.01 |
||||||||||||||
| Crystal Structure | NaCl type |
||||||||||||||
| crystal system | Cube |
||||||||||||||
| Merck | 14,8599 |
||||||||||||||
| BRN | 3534976 |
||||||||||||||
| Space group | Fm3m |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Dielectric constant | 5.9(Ambient) |
||||||||||||||
| BCS Class | 1 |
||||||||||||||
| Stability: | Stable. Incompatible with strong oxidizing agents. |
||||||||||||||
| InChIKey | FAPWRFPIFSIZLT-UHFFFAOYSA-M |
||||||||||||||
| CAS DataBase Reference | 7647-14-5(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Sodium chloride(7647-14-5) |
||||||||||||||
| EPA Substance Registry System | Sodium chloride (7647-14-5) |
||||||||||||||
| Absorption | ≤0.01 at 260 ≤0.01 at 280 in H2O at 1M |
Description and Uses
Sodium chloride is widely distributed in nature. Oceans are the vast source of sodium chloride. It occurs in seawater at an average concentration of 2.68 wt%. It also occurs in many inland saline waters and in salt deposits in sedimentary rocks, as the mineral halite.
Sodium chloride is probably the most important salt of both sodium and chlorine. Sodium chloride, common table salt, is an essential component of most food preparation, imparting flavor to food and providing the sodium nutritional requirement. Also, it is used for preserving food. Therapeutically, NaCl solution is used to combat dehydration as an electrolyte replenisher, and it is an emetic.
The most important applications of sodium chloride in the chemical industry are in making a number of important industrial chemicals such as hydrochloric acid, sodium hydroxide, sodium carbonate, and metallic sodium. It is the starting material in manufacturing these substances. Other uses are in dyeing and printing fabrics, glazing pottery, in making soap, and for curing hides. Sodium chloride is a component of many freezing mixtures.
Natural salt is the source of chlorine and of sodium as well as of all, or practically all, their Compounds, e.g., hydrochloric acid, chlorates, sodium carbonate, hydroxide, etc.; for preserving foods; manufacture of soap, to salt out dyes; in freezing mixtures; for dyeing and printing fabrics, glazing pottery, curing hides; metallurgy of tin and other metals.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H303 |
| Precautionary statements | P270-P301+P312-P403-P501c |
| Hazard Codes | Xi |
| Risk Statements | 36-36/37/38-22 |
| Safety Statements | 24/25-26-36 |
| WGK Germany | 1 |
| RTECS | VZ4725000 |
| F | 3-10 |
| TSCA | Yes |
| HS Code | 38220000 |
| Hazardous Substances Data | 7647-14-5(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 3.75 ±0.43 g/kg (Boyd, Shanas) |