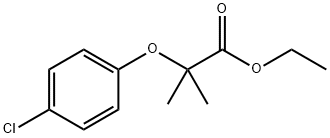
Clofibrate , 98% , 637-07-0
Synonym(s):
2-(4-Chlorophenoxy)-2-methylpropionic acid ethyl ester;Ethyl 2-(4-chlorophenoxy)-2-methylpropionate;Ethyl 2-(4-chlorophenoxy)isobutyrate
CAS NO.:637-07-0
Empirical Formula: C12H15ClO3
Molecular Weight: 242.7
MDL number: MFCD00000615
EINECS: 211-277-4
| Pack Size | Price | Stock | Quantity |
| 5g | RMB63.20 | In Stock |
|
| 25G | RMB126.40 | In Stock |
|
| 100G | RMB523.20 | In Stock |
|
| 500G | RMB1919.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | <25 °C |
| Boiling point: | 154 °C |
| Density | 1.14 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 113 °C |
| storage temp. | 2-8°C |
| solubility | Soluble to 100 mM in DMSO. |
| form | liquid |
| color | clear, colorless |
| Water Solubility | 97.08mg/L(room temperature) |
| Merck | 14,2377 |
| BRN | 1913459 |
| CAS DataBase Reference | 637-07-0(CAS DataBase Reference) |
| IARC | 3 (Vol. Sup 7, 66) 1996 |
| NIST Chemistry Reference | Clofibrate(637-07-0) |
| EPA Substance Registry System | Clofibrate (637-07-0) |
Description and Uses
Researchers in France observed in 1953 that structures derived
from dehydrocholic acid, phenylethyl acetic acid, and certain
other disubstituted acetic acids exhibited hypocholesterolemic
properties in rats and humans. Several years later, Thorp and
Waring discovered clof ibrate as an effective compound for
lowering lipids in animal models, with minimal toxicity. Its
mode of action was initially attributed to seasonal variations in
adrenal and thyroid function, and the administration of
androsterone was found to potentiate the hypocholesterolemic
effect of this compound. Subsequently, several clinical trials
were performed which showed that clofibrate decreases lipid
levels in hypercholesterolemic patients, mainly as the result of
a reduction in the very-low-density lipoprotein (VLDL), and
less in the low-density lipoprotein (LDL) fraction, and that the
coadministration of androsterone was not necessary for its
hypolipidemic effect. Despite reported hepatomegaly in rats
following long-term treatment with clofibrate, this drug was
approved in the United States in 1967 for the treatment of
hyperlipidemias.
Clofibrate can be chemically synthesized by the condensation
of phenol with ethyl 2-chloro-2-methylpropionate in the
presence of a dehydrochlorinating agent, followed by chlorination
and purification. It can also be synthesized by the
condensation of p-chlorophenol with acetone and chloroform
followed by esterifying the resultant acid to give clofibrate.
inhibits cholesterol biosynthesis
Safety
| Symbol(GHS) |   GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302-H315-H318-H335 |
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn,N |
| Risk Statements | 22-37/38-41-51/53-40 |
| Safety Statements | 26-36/37/39-61-45-36/37 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| RTECS | UE9480000 |
| HazardClass | 9 |
| HS Code | 29189900 |
| Hazardous Substances Data | 637-07-0(Hazardous Substances Data) |
| Toxicity | LD50 in mice, rats (g/kg): 1.28, 1.65 orally (Metz) |