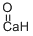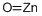Calcium oxide , 99.95%metalsbasis , 1305-78-8
Synonym(s):
Calcium oxide from marble;Lime;Lime, caustic, Quicklime;Quicklime
CAS NO.:1305-78-8
Empirical Formula: CaO
Molecular Weight: 56.08
MDL number: MFCD00010911
EINECS: 215-138-9
| Pack Size | Price | Stock | Quantity |
| 10G | RMB159.20 | In Stock |
|
| 50G | RMB639.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 2570 °C |
||||||||||||||
| Boiling point: | 2850 °C (lit.) |
||||||||||||||
| Density | 3.3 g/mL at 25 °C (lit.) |
||||||||||||||
| bulk density | 800-1200kg/m3 |
||||||||||||||
| refractive index | 1.83 |
||||||||||||||
| Flash point: | 2850°C |
||||||||||||||
| storage temp. | no restrictions. |
||||||||||||||
| solubility | 1.65g/l Risk of violent reaction. |
||||||||||||||
| form | powder |
||||||||||||||
| color | White to yellow-very slightly beige |
||||||||||||||
| Specific Gravity | 3.3 |
||||||||||||||
| PH | 12.6 (H2O, 20℃)(saturated solution) |
||||||||||||||
| Odor | wh. or gray cryst. or powd., odorless |
||||||||||||||
| Water Solubility | REACTS |
||||||||||||||
| Sensitive | Air & Moisture Sensitive |
||||||||||||||
| Crystal Structure | Cubic |
||||||||||||||
| crystal system | Cube |
||||||||||||||
| Merck | 14,1686 |
||||||||||||||
| Space group | Fm3m |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Dielectric constant | 2.2(Ambient) |
||||||||||||||
| Exposure limits | ACGIH: TWA 2 mg/m3 OSHA: TWA 5 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3 |
||||||||||||||
| Stability: | Stability Stable, but absorbs carbon dioxide from the air. Incompatible with water, moisture, fluorine, strong acids. |
||||||||||||||
| InChIKey | ODINCKMPIJJUCX-UHFFFAOYSA-N |
||||||||||||||
| CAS DataBase Reference | 1305-78-8(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Calcium monoxide(1305-78-8) |
||||||||||||||
| EPA Substance Registry System | Calcium oxide (1305-78-8) |
Description and Uses
Calcium oxide (CaO, CAS Reg. No. 1305-78-8) is also known as lime, quick lime, burnt lime, or calx. Lime does not occur naturally since it reacts so readily with water (to form hydrated lime) and carbon dioxide (to form limestone). It is produced from calcium carbonate, limestone, or oyster shells by calcination at temperatures of 1,700-2,450℃.
Calcium Oxide is a solid with a very high affinity for water - it will react with water in the air, or in your skin or anywhere it can and form calcium hydroxide. This reaction is exothermic so it releases a lot of heat while it is reacting - there fore as well as being corrosive and causing significant skin irritation, calcium oxide's reaction with water can also cause burns. Calcium hydroxide is basically hydrated calcium oxide. It is alkali so can be corrosive. In solution it makes limewater.
CaO is not found pure in nature but rather is contained in various abundant minerals (i.e. calcite, aragonite, limestone, marble) but vary greatly in their purity (impurities usually include magnesia, iron, alumina, silica, sulfur). Of these iron and sulfur are most troublesome (i.e. where clarity is important in glass). Lime minerals vary in the degree of crystallization and cohesion of the crystalline mass and the homogeneity of the matrix.
Calcium oxide is the principle flux in medium and high temperature glazes, beginning its action (within the glaze) around 1100C. It must be used with care in high-fire bodies because its active fluxing action can produce a body that is too volatile (melting if slightly overfired).
Lime, or calcium oxide, is a principle ingredient in the production of Portland cement, the basis for most mortars and concrete. Hydrated or ‘slaked’ lime is the chemical calcium hydroxide. This chemical is also used in mortars. Both types of lime are strong bases and are also used in food production (calcium hydroxide is commonly used in making corn tortillas), petroleum refining and sewage treatment. In the household it is used by aquarium hobbyists to add bioavailable calcium to fish tanks. It is also found in hair relaxers.
The major uses of lime are metallurgy, flue gas desulfurization, construction, mining, papermaking, and water treatment. About one third of calcium oxide production in the United States is used for metallurgical processes, principally in the iron and steel industry. Calcium oxide is used to remove impurities during the refining of iron ore. Calcium oxide combines with compounds such as silicates, phosphates, and sulfates contained in iron ores to form slag. Lime is also used for purification in other metal refining and to control pH in mining processes such as leaching and precipitation. The calcium oxide is also used in remediation of mine wastes to recover cyanides and to neutralize acid mine drainage.
Safety
| Symbol(GHS) |   GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H315-H318-H335 |
| Precautionary statements | P280-P302+P352-P305+P351+P338+P310 |
| Hazard Codes | C,Xi |
| Risk Statements | 34-41-37/38 |
| Safety Statements | 26-36/37/39-45-25-39 |
| RIDADR | 1910 |
| OEB | B |
| OEL | TWA: 2 mg/m3 |
| WGK Germany | 1 |
| RTECS | EW3100000 |
| F | 10-21-34 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 28259019 |
| Hazardous Substances Data | 1305-78-8(Hazardous Substances Data) |
| IDLA | 25 mg/m3 |