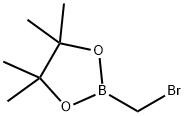
2-(Bromomethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane , 90% , 166330-03-6
| Pack Size | Price | Stock | Quantity |
| 1G | RMB134.40 | In Stock |
|
| 5G | RMB399.20 | In Stock |
|
| 25g | RMB1356.00 | In Stock |
|
| 100g | RMB3823.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Boiling point: | 68 °C / 6mmHg |
| Density | 1.26 |
| refractive index | 1.4520-1.4560 |
| Flash point: | 62.8±22.6℃ |
| storage temp. | Inert atmosphere,2-8°C |
| form | clear liquid |
| color | Colorless to Light orange to Yellow |
| InChI | InChI=1S/C7H14BBrO2/c1-6(2)7(3,4)11-8(5-9)10-6/h5H2,1-4H3 |
| InChIKey | KBGMAXNDJAGTDD-UHFFFAOYSA-N |
| SMILES | O1C(C)(C)C(C)(C)OB1CBr |
| CAS DataBase Reference | 166330-03-6 |
Description and Uses
(Bromomethyl)boronic Acid Pinacol Ester, also known as 2-(Bromomethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (BMTMD), is an organoboron compound extensively researched for its potential applications in organic synthesis and catalysis. It is a versatile reagent, exhibiting a broad range of reactivity suitable for various reactions, including Diels-Alder, Michael, and Wittig reactions. Moreover, BMTMD proves valuable as a catalyst in the synthesis of diverse compounds like heterocycles, amines, polymers (such as polyesters and polyamides), and organometallic compounds (including boronates, boronic esters, and boronic acids). Its ability to react with various functional groups, including carbonyl and carboxyl, facilitates nucleophilic addition and substitution mechanisms. Additionally, BMTMD can interact with amines, alcohols, and sulfonates, where nucleophilic addition and substitution mechanisms are involved depending on the functional group.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H227-H315-H319 |
| Precautionary statements | P210-P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313-P403+P235-P501 |
| HS Code | 2931900090 |