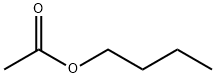
Butyl acetate , AR,99.0% , 123-86-4
Synonym(s):
n-Butyl acetate;Acetic acid n-butyl ester, Butyl ethanoate;butanol;Butyl acetate;Butyl ethanoate
CAS NO.:123-86-4
Empirical Formula: C6H12O2
Molecular Weight: 116.16
MDL number: MFCD00009445
EINECS: 204-658-1
PRODUCT Properties
| Melting point: | -78 °C (lit.) |
| Boiling point: | 124-126 °C (lit.) |
| Density | 0.88 g/mL at 25 °C (lit.) |
| vapor density | 4 (vs air) |
| vapor pressure | 15 mm Hg ( 25 °C) |
| refractive index | n |
| FEMA | 2174 | BUTYL ACETATE |
| Flash point: | 74 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 5.3g/l |
| form | Liquid |
| Specific Gravity | 0.883 (20/20℃) |
| color | ≤10(APHA) |
| Odor | Characteristic; agreeable fruity (in low concentrations); non residual. |
| PH | 6.2 (5.3g/l, H2O, 20℃)(External MSDS) |
| explosive limit | 1.4-7.5%(V) |
| Odor Threshold | 0.016ppm |
| Odor Type | ethereal |
| biological source | synthetic |
| Water Solubility | 0.7 g/100 mL (20 ºC) |
| FreezingPoint | -77.9℃ |
| λmax | λ: 254 nm Amax: 1.0 λ: 260 nm Amax: 0.20 λ: 275 nm Amax: 0.04 λ: 300 nm Amax: 0.02 λ: 320-400 nm Amax: 0.01 |
| JECFA Number | 127 |
| Merck | 14,1535 |
| BRN | 1741921 |
| Henry's Law Constant | 5.79 at 37 °C (static headspace-GC, van Ruth et al., 2001) |
| Exposure limits | TLV-TWA 150 ppm (~710 mg/m3) (ACGIH,
MSHA, and OSHA); TLV-STEL 200 ppm
(~950 mg/m3); IDLH 10,000 ppm (NIOSH). |
| Dielectric constant | 5.0(20℃) |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, strong acids, strong bases. |
| InChIKey | DKPFZGUDAPQIHT-UHFFFAOYSA-N |
| LogP | 1.82-2.3 at 25℃ |
| Surface tension | 24.85mN/m at 298.15K |
| CAS DataBase Reference | 123-86-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, butyl ester(123-86-4) |
| EPA Substance Registry System | n-Butyl acetate (123-86-4) |
Description and Uses
Butyl acetate is a clear, flammable ester of acetic acid that occurs in n-, sec-, and tert- forms (INCHEM, 2005). Butyl acetate isomers have a fruity, banana-like odor (Furia, 1980). Isomers of butyl acetate are found in apples (Nicholas, 1973) and other fruits (Bisesi, 1994), as a well as in a number of food products, such as cheese, coffee, beer, roasted nuts, vinegar (Maarse and Visscher, 1989). Butyl acetate is manufactured via esterification of the respective alcohol with acetic acid or acetic anhydride (Bisesi, 1994). N-butyl acetate is used as a solvent for nitrocellulose-based lacquers, inks, and adhesives. Other uses include manufacture of artificial leathers, photographic film, safety glass, and plastics (Budavari, 1996). Isomers of butyl acetate are also used as flavoring agents, in manicure products, and as larvicides (Bisesi, 1994). The tert-isomer has been used as a gasoline additive (Budavari, 1996). It may be used as a synthetic fruit flavoring in candy, ice cream, cheeses, and baked goods (Dikshith, 2013).
Butyl acetate is one of the more important derivatives of n-butyl alcohol produced commercially, is employed as a solvent in rapid drying paints and coatings. In some instances, butyl acetate, C6H12O2, has replaced ethoxyethyl acetate due to the latter’s reported toxicity and teratogenicity.
Safety
| Symbol(GHS) |   GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226-H336 |
| Precautionary statements | P210 |
| Risk Statements | 10-66-67-R67-R66-R10 |
| Safety Statements | 25-S25 |
| OEB | A |
| OEL | TWA: 150 ppm (710 mg/m3), STEL: 200 ppm (950 mg/m3) |
| RIDADR | UN 1123 3/PG 3 |
| WGK Germany | 1 |
| RTECS | AF7350000 |
| Autoignition Temperature | 790 °F |
| TSCA | Yes |
| HS Code | 2915 33 00 |
| HazardClass | 3 |
| PackingGroup | III |
| Hazardous Substances Data | 123-86-4(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 14.13 g/kg (Smyth) |
| IDLA | 1,700 ppm |