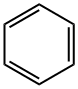
Benzene , standardforGC,≥99.9%(GC) , 71-43-2
Synonym(s):
Benzene;Benzene in dimethyl sulfoxide;Benzene solution;Benzine;Benzol
CAS NO.:71-43-2
Empirical Formula: C6H6
Molecular Weight: 78.11
MDL number: MFCD00003009
EINECS: 200-753-7
PRODUCT Properties
| Melting point: | 5.5 °C (lit.) |
| Boiling point: | 80 °C (lit.) |
| Density | 0.874 g/mL at 25 °C (lit.) |
| vapor density | 2.77 (vs air) |
| vapor pressure | 166 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | 12 °F |
| storage temp. | room temp |
| solubility | Miscible with alcohol, chloroform, dichloromethane, diethyl ether, acetone and acetic acid. |
| pka | 43(at 25℃) |
| form | Liquid |
| color | APHA: ≤10 |
| Relative polarity | 0.111 |
| Odor | Paint-thinner-like odor detectable at 12 ppm |
| Odor Threshold | 2.7ppm |
| explosive limit | 1.4-8.0%(V) |
| Water Solubility | 0.18 g/100 mL |
| λmax | λ: 280 nm Amax: 1.0 λ: 290 nm Amax: 0.15 λ: 300 nm Amax: 0.06 λ: 330 nm Amax: 0.02 λ: 350-400 nm Amax: 0.01 |
| Merck | 14,1066 |
| BRN | 969212 |
| Henry's Law Constant | 10.4 at 45.00 °C, 11.4 at 50.00 °C, 13.3 at 55.00 °C, 14.5 at 60.00 °C, 16.8 at 65.00 °C, 19.2 at
70.00 °C (static headspace-GC, Park et al., 2004) |
| Exposure limits | TLV-TWA 10 ppm (~32 mg/m3) (ACGIH
and OSHA); ceiling 25 ppm (~80 mg/m3)
(OSHA and MSHA); peak 50 ppm (~160
mg/m3)/10 min/8 h (OSHA); carcinogenicity:
Suspected Human Carcinogen (ACGIH),
Human Sufficient Evidence (IARC). |
| Dielectric constant | 2.3(20℃) |
| Stability: | Stable. Substances to be avoided include strong oxidizing agents, sulfuric acid, nitric acid, halogens. Highly flammable. |
| InChIKey | UHOVQNZJYSORNB-UHFFFAOYSA-N |
| LogP | 2.130 |
| Surface tension | 28.891mN/m at 293.15K |
| CAS DataBase Reference | 71-43-2(CAS DataBase Reference) |
| IARC | 1 (Vol. 29, Sup 7. 100F, 120) 2018 |
| NIST Chemistry Reference | Benzene(71-43-2) |
| EPA Substance Registry System | Benzene (71-43-2) |
Description and Uses
Benzene is a colorless, volatile, highly flammable liquid that is used extensively in the chemical industry and received wide interest in the early days of organic chemistry.
Because of its structure, benzene is a very stable organic compound. It does not readily undergo addition reactions. Addition reactions involving benzene require high temperature, pressure, and special catalysts. The most common reactions involving benzene involve substitution reactions. Numerous atoms and groups of atoms may replace a hydrogen atom or several hydrogen atoms in benzene. Th ree important types of substitution reactions involving benzene are alkylation, halogenation, and nitration. In alkylation, an alkyl group or groups substitute for hydrogen(s).
Benzene is also converted to cyclohexane, which is used to produce nylon and synthetic fibers.
Safety
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H225-H304-H315-H319-H340-H350-H372-H412 |
| Precautionary statements | P210-P273-P301+P310-P303+P361+P353-P305+P351+P338-P331 |
| Hazard Codes | F,T |
| Risk Statements | 45-46-11-36/38-48/23/24/25-65-39/23/24/25-23/24/25 |
| Safety Statements | 53-45-36/37-16-7 |
| RIDADR | UN 1114 3/PG 2 |
| OEB | E |
| OEL | TWA: 0.1 ppm, STEL: 1 ppm |
| WGK Germany | 3 |
| RTECS | CY1400000 |
| F | 3-10 |
| Autoignition Temperature | 560 °C |
| TSCA | Yes |
| HS Code | 2902 20 00 |
| HazardClass | 3 |
| PackingGroup | II |
| Hazardous Substances Data | 71-43-2(Hazardous Substances Data) |
| Toxicity | LD50 orally in young adult rats: 3.8 ml/kg (Kimura) |
| IDLA | 500 ppm |