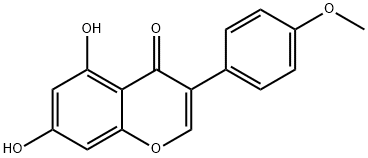
Biochanin A , Analysis standard , 491-80-5
Synonym(s):
4′-Methylgenistein;5,7-Dihydroxy-4′-methoxyisoflavone;Genistein 4′-methyl ether;Olmelin
CAS NO.:491-80-5
Empirical Formula: C16H12O5
Molecular Weight: 284.26
MDL number: MFCD00006839
EINECS: 207-744-7
| Pack Size | Price | Stock | Quantity |
| 50MG | RMB156.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 210-213 °C(lit.) |
| Boiling point: | 340-355 °C(Press: 0.5 Torr) |
| Density | 1.420±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | acetone: 10 mg/mL, clear, brown |
| pka | 6.50±0.20(Predicted) |
| form | Powder |
| color | Off-White to Beige |
| Water Solubility | Soluble in water (<1 mg/ml at 25°C), chloroform, methanol, DMSO (57 mg/ml at 25°C), and ethanol (9 mg/ml at 25°C). |
| λmax | 263nm(EtOH)(lit.) |
| BRN | 278107 |
| LogP | 3.341 (est) |
| CAS DataBase Reference | 491-80-5(CAS DataBase Reference) |
| EPA Substance Registry System | 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-methoxyphenyl)- (491-80-5) |
Description and Uses
Biochanin A is a natural isoflavone with diverse biological actions, most notably as a phytoestrogen. It can affect hormone levels by inhibiting 5α-reductase and 17β-hydroxysteroid dehydrogenase or altering aromatase (CYP19A1) activity. Also known as 4’-methyl genistein, biochanin A can be metabolized in vivo to genistein , another phytoestrogen with diverse effects. Biochanin A also intersects with signaling through peroxisome proliferator-activated receptors (PPARs), as it activates PPARγ (EC50 = 19 μM) and has also been shown to activate a PPARα promoter. Moreover, it increases the expression of the PPARγ coactivator PGC-1α, promoting mitochondrial biogenesis. Biochanin A also inhibits fatty acid amide hydrolase (IC50 = 2.4 μM) and acts as an agonist of the aryl hydrocarbon receptor (EC50 = 0.25 μM).
It has putative benefits in dietary cancer prophylaxis. It has also been found to inhibit fatty acid amide hydrolase and to act as agonist of PPARgamma, nuclear receptor that is current pharmacological target for the treatment of diabetes type 2. It acts as an antineoplastic agent. It is a selective agonist at ER-β estrogen receptors, and may have chemopreventive efficacy against breast cancer. In line with its low activity at ER-α estrogen receptors, it is essentially devoid of uterotrophic activity. Biochanin A is also a ligand for the aryl hydrocarbon receptor (AhR). It reduces arterial resistance and enhances microcirculation perhaps via effects on potassium and/or calcium ion channels. Induction of sulfotransferases for xenobiotic detoxification has been proposed as a mechanism of its cancer preventive effects. It is a nitric oxide synthase inhibitor and apoptosis inducer