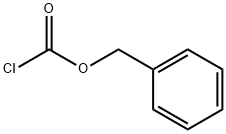
Benzyl chloroformate , 96%, containing about 0.1%sodium carbonate stabilizer , 501-53-1
Synonym(s):
Benzyl chlorocarbonate, Carbonochloride acid benzylester;Benzyl chloroformate;Carbobenzoxy chloride;Z-Chloride
CAS NO.:501-53-1
Empirical Formula: C8H7ClO2
Molecular Weight: 170.59
MDL number: MFCD00000640
EINECS: 207-925-0
PRODUCT Properties
| Melting point: | -20 °C |
| Boiling point: | 103 °C20 mm Hg(lit.) |
| Density | 1.212 g/mL at 20 °C |
| vapor density | 1 (vs air) |
| vapor pressure | 1.39 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 197 °F |
| storage temp. | Store at +2°C to +8°C. |
| solubility | Miscible with ether, acetone and benzene. |
| form | Solution |
| color | slightly yellow to slightly pink |
| Odor | Irritating; sharp, penetrating. |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,1801 |
| Exposure limits | ACGIH: TWA 1 ppm OSHA: TWA 1 ppm(5 mg/m3) NIOSH: IDLH 10 ppm; Ceiling 1 ppm(5 mg/m3) |
| Stability: | Stable. Incompatible with water, oxidizing agents. Combustible. |
| CAS DataBase Reference | 501-53-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzyl chloroformate(501-53-1) |
| EPA Substance Registry System | Carbonochloridic acid, phenylmethyl ester (501-53-1) |
Description and Uses
Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl (Cbz or Z) group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.
The compound was first prepared by Leonidas Zervas in the early 1930s who used it for the introduction of the benzyloxycarbonyl protecting group, which became the basis of the Bergmann-Zervas carboxybenzyl method of peptide synthesis he developed with Max Bergmann. This was the first successful method of controlled peptide chemical synthesis and for twenty years it was the dominant procedure used worldwide until the 1950s. To this day, benzyl chloroformate is often used for amine group protection.
Benzyl chloroformate is widely used as a reactive chemical intermediate in plastic, pharmaceutical, agricultural and organic chemicals. It is useful for the introduction of carboxybenzyl (cbz) protecting group for amines such as aniline in organic synthesis. It is also involved in the synthesis of 1,2,4-oxadiazoles.
Safety
| Symbol(GHS) |     GHS05,GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H314-H335-H350-H410 |
| Precautionary statements | P201-P261-P273-P280-P305+P351+P338-P310 |
| Hazard Codes | T,C,N,F |
| Risk Statements | 45-20-34-48/22-50/53-67-65-63-48/20-11-43-37-23 |
| Safety Statements | 53-26-36/37/39-45-60-61-62-46-36/37 |
| RIDADR | UN 2924 3/PG 2 |
| WGK Germany | 3 |
| RTECS | LQ5860000 |
| F | 19 |
| Autoignition Temperature | 590 °C |
| TSCA | Yes |
| HS Code | 2915 90 70 |
| HazardClass | 8 |
| PackingGroup | I |
| Hazardous Substances Data | 501-53-1(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 3000 mg/kg |