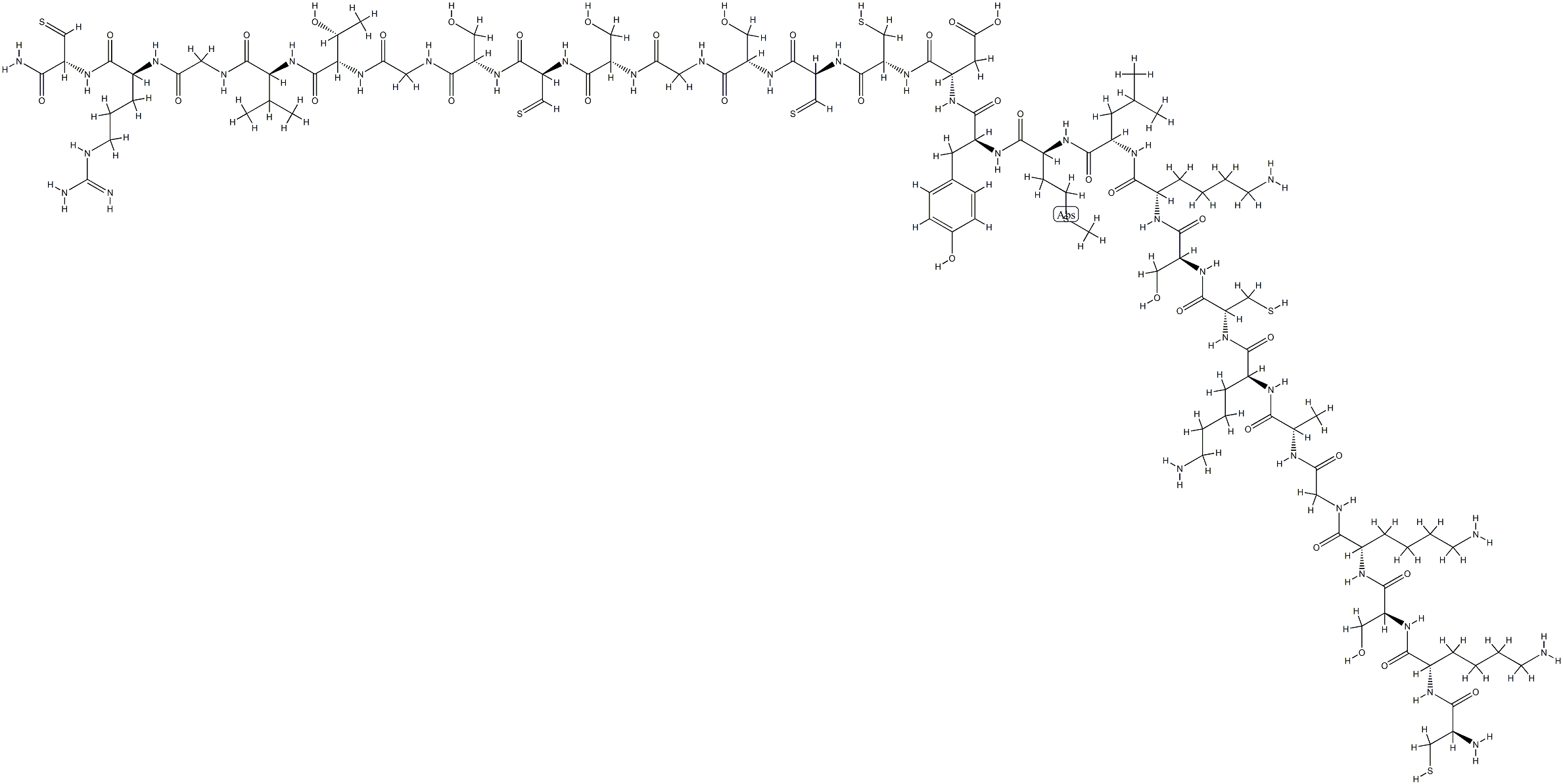
Cetuximab, a human/mouse chimeric monoclonal antibody that blocks the EGFR,
was launched for use in combination with irinotecan in the treatment of patients with colorectal cancer who no longer respond to standard chemotherapy
treatment with irinotecan. Cetuximab is obtained by chimerization of M225, a
murine anti-EGFR antibody; specifically heavy and light chains of the murine
antibody are cloned and adapted for expression with constant regions of the human
kappa light chain and human gamma1 heavy chain. It is produced by mammalian
(suspension) cells in serum-free medium, purified by protein A affinity chromatography,
ion-exchange chromatography, and gel filtration. Cetuximab binds specifically
to EGFR on both normal and tumor cells. Overexpression of the human EGFR is
detected in many cancers, including those of the colon and rectum. The binding of
cetuximab to EGFR prevents growth factors from binding to the receptor, thereby
inhibiting cell growth and inducing apoptosis. The therapeutic regimen of cetuximab
consists of an initial loading dose of 400 mg/m2 administered as a 120-min IV
infusion, followed by weekly maintenance dose of 250 mg/m2 infused over 60 min.
The steady-state plasma concentrations of cetuximab are reached by the third weekly
infusion and the mean elimination half-life is 114 h. Cetuximab is eliminated by
binding to EGFRs in various tissues, followed by internalization of the antibody-
EGFR complex. Systemic clearance of the antibody is saturated at higher doses, and
this appears to correlate with saturation of EGFR binding. In a multicenter clinical
trial in more than 300 patients with advanced metastatic colorectal cancer,
combination therapy with cetuximab and irinotecan produced response in more
than half of the patients, shrinking tumors in 23% and stopping tumor growth in an
additional 33% of the patients. The most common adverse reactions associated with
cetuximab were acneform rash, asthenia/malaise, fever, nausea, abdominal pain,
constipation and vomiting. Serious adverse events such as infusion reaction, fever,
sepsis, kidney failure, dehydration and diarrhea were experienced by <10% of the
patients.
Treatment of EGF receptor-expressing cancers
(monoclonal antibod.