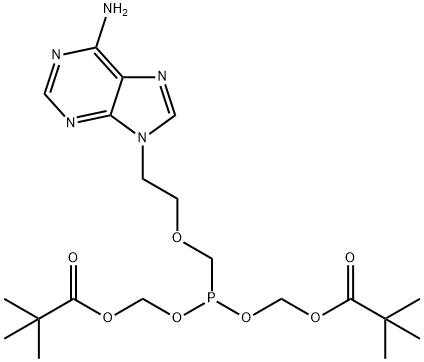
Adefovir Dipivoxil , ≥99% , 142340-99-6
Synonym(s):
9-(2[bis(Pivaloyloxymethoxy)phosphorylmethoxy]ethyl)adenine
CAS NO.:142340-99-6
Empirical Formula: C20H32N5O8P
Molecular Weight: 501.47
MDL number: MFCD00869897
EINECS: 200-001-8
| Pack Size | Price | Stock | Quantity |
| 25MG | RMB23.20 | In Stock |
|
| 100MG | RMB46.40 | In Stock |
|
| 250MG | RMB70.40 | In Stock |
|
| 1G | RMB86.40 | In Stock |
|
| 5G | RMB236.00 | In Stock |
|
| 25G | RMB780.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 98-102°C |
| Boiling point: | 641.0±65.0 °C(Predicted) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | ethanol: soluble50mg/mL |
| pka | 4.16±0.10(Predicted) |
| form | solid |
| color | White to Off-White |
| biological source | synthetic |
| Merck | 14,151 |
| InChIKey | WOZSCQDILHKSGG-UHFFFAOYSA-N |
| CAS DataBase Reference | 142340-99-6(CAS DataBase Reference) |
Description and Uses
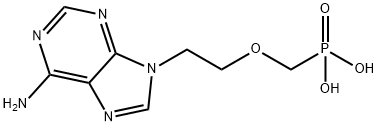
Adefovir dipivoxil is the first nucleotide analog to be launched in the US as an oral treatment for hepatitis B virus (HBV) infections. It can be easily prepared in 4 steps from adenine. Adefovir dipivoxil acts as a bioavailable ester prodrug which is rapidly hydrolyzed to free adefovir and further anabolized to its active form, adefovir diphosphate, by two intracellular phosphotylation steps. The diphosphate competitively inhibits reverse transcriptase and/or causes chain termination when incorporated into growing DNA. Adefovir dipivoxil has a broad antiviral spectrum against retro-, herpes- and hepadnaviruses. The drug inhibits HBV replication, decreases HBV DNA levels and improves liver histology of patients infected with HBV wild type and resistant to other antivirals such as lamivudine. It also demonstrated activity in hepatitis B”e” antigennegative, or precore mutant, patients and in patients co-infected with HIV. To date, no adefovir dipivoxil-associated resistance mutations have been identified in patients up to 136 weeks with the drug. The oral bioavailability of adefovir after oral administration of its dipivoxil prodrug is approximately 30%. It is mainly excreted unchanged in the urine and its plasma elimination half-life is 4.2 h. However, a long intracellular half-life (17 h) of the active bisphosphorylated metabolite enables once-daily dosing. The most prominent adverse effect of adefovir dipivoxil is nephrotoxicity (which has prevented the drug from being marketed for HIV infections where the drug required administration at higher doses).
Adefovir Dipivoxil(Preveon, Hepsera) works by blocking reverse transcriptase, an enzyme that is crucial for the hepatitis B virus (HBV) to reproduce in the body. It is approved for the treatment of chronic hepatitis B in adults with evidence of active vir
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302+H312+H332 |
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352+P312-P304+P340+P312 |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22 |
| Safety Statements | 36/37 |
| WGK Germany | 3 |
| RTECS | UA2459362 |
| HS Code | 2933.99.8290 |

![Diethyl [[2-(6-Amino-9<i>H</i>-purin-9-yl)ethoxy]methyl]phosphonate](https://img.chemicalbook.com/CAS/GIF/116384-53-3.gif)
![Propanoic acid, 2,2-dimethyl-, [[[[2-(6-amino-9H-purin-9-yl)ethoxy]methyl]ethoxyphosphinyl]oxy]methyl ester](https://img.chemicalbook.com/CAS/GIF/142341-04-6.gif)