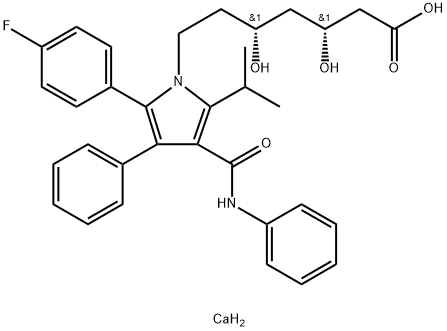
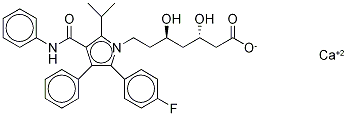
Atorvastatin calcium salt trihydrate , 98% , 134523-03-8
Synonym(s):
(3 R,5 R)-7-(2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate, hemicalcium salt;(3R,5R)-7-(2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate, hemicalcium salt;(betaR,deltaR)-2-(p-Fluorophenyl)-beta,delta-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoate, calcium salt (2:1) trihyddrate;[R-(R*, R*)]-2-(4-Fluorophenyl)-β, δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1Hpyrrole-1-heptanoic acid, calcium salt (2:1) trihydrate;1H-Pyrrole-1-heptanoic acid, [R-(R*,R*)]-2-(4-flurophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl], calcium salt (2:1)
CAS NO.:134523-03-8
Empirical Formula: C66H68CaF2N4O10
Molecular Weight: 1155.36
MDL number: MFCD03613598
EINECS: 200-659-6
| Pack Size | Price | Stock | Quantity |
| 250MG | RMB56.00 | In Stock |
|
| 1G | RMB152.00 | In Stock |
|
| 5g | RMB535.20 | In Stock |
|
| 10g | RMB692.00 | In Stock |
|
| 50g | RMB1813.60 | In Stock |
|
| 100g | RMB2957.60 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 176-178°C |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO: ≥10mg/mL |
| form | white powder |
| color | White to Off-White |
| BCS Class | 2 |
| InChIKey | FQCKMBLVYCEXJB-MNSAWQCASA-L |
| SMILES | C(C1=C(N(CC[C@@H](O)C[C@@H](O)CC(=O)O)C(C2C=CC(F)=CC=2)=C1C1C=CC=CC=1)C(C)C)(=O)NC1C=CC=CC=1.[Ca] |&1:6,9,r| |
| CAS DataBase Reference | 134523-03-8(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-.beta.,delta.-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-, calcium salt (2:1),(.beta.R,.delta.R)- (134523-03-8) |
Description and Uses
Lipitor was launched in Canada, the Netherlands, the UK and the US as an orally-active hypocholesterolemic agent. It was the first pharmaceutical product ever to attain over one billion dollars in sales in its first year. It can be synthesized by a number of routes but the most efficient involves the Paal-Knorr reaction of an acetonide protected dihydroxy amino ester and a diaryl phenylacetamide diketone. Lipitor is a liver selective, reversible competitive inhibitor of HMG-CoA reductase, the rate limiting step in cholesterol biosynthesis. Lipitor monotherapy resulted in a reduction of LDL cholesterol by up to 60%. Lipitor is about 2-4 times more potent, on a dosage basis, than Simvastatin. The superior properties of Lipitor can be attributed to its greater uptake and longer duration of action in the liver. In addition to its effects on cholesterol, Lipitor is also effective in lowering triglycerides. While the mechanism is not clear, two theories proposed are: a) the decrease in cholesterol causes a concomitant increase in hepatic LDL-receptor activity (mostly B and E type) which results in a decrease in triglycerides through an increase in binding of triglycerides to VLDL and LDL, and b) the decreased level of cholesterol impairs VLDL transport of triglycerides.
The primary uses of atorvastatin is for the treatment of dyslipidemia and the prevention of cardiovascular disease.
Safety
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
| Signal word | Warning |
| Hazard statements | H411-H351-H361f-H372-H319 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P260-P264-P270-P314-P501-P264-P280-P305+P351+P338-P337+P313P |
| Hazard Codes | Xn,T,F |
| Risk Statements | 20/21/22-36/37/38-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-36-45-36/37-16-7 |
| WGK Germany | 3 |