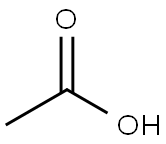
Acetate , forLC-MS , 64-19-7
Synonym(s):
Glacial acetic acid;Acetic acid;aa;Ethanoic acid;Acetic acid solution
CAS NO.:64-19-7
Empirical Formula: C2H4O2
Molecular Weight: 60.05
MDL number: MFCD00011354
EINECS: 200-580-7
PRODUCT Properties
| Melting point: | 16.2 °C(lit.) |
| Boiling point: | 117-118 °C(lit.) |
| Density | 1.049 g/mL at 25 °C(lit.) |
| vapor density | 2.07 (vs air) |
| vapor pressure | 11.4 mm Hg ( 20 °C) |
| FEMA | 2006 | ACETIC ACID |
| refractive index | n |
| Flash point: | 104 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: miscible(lit.) |
| form | Solution |
| pka | 4.74(at 25℃) |
| Specific Gravity | 1.0492 (20℃) |
| color | colorless |
| Odor | Strong, pungent, vinegar-like odor detectable at 0.2 to 1.0 ppm |
| PH | 3.91(1 mM solution);3.39(10 mM solution);2.88(100 mM solution); |
| PH Range | 2.4 (1.0M solution) |
| Odor Threshold | 0.006ppm |
| Odor Type | acidic |
| explosive limit | 4-19.9%(V) |
| biological source | synthetic |
| Water Solubility | miscible |
| λmax | λ: 260 nm Amax: 0.05 λ: 270 nm Amax: 0.02 λ: 300 nm Amax: 0.01 λ: 500 nm Amax: 0.01 |
| Merck | 14,55 |
| JECFA Number | 81 |
| BRN | 506007 |
| Henry's Law Constant | 133, 122, 6.88, and 1.27 at pH values of 2.13, 3.52, 5.68, and 7.14, respectively (25 °C, Hakuta et
al., 1977) |
| Exposure limits | TLV-TWA 10 ppm ~25 mg/m3) (ACGIH,
OSHA, and MSHA); TLV-STEL 15 ppm
(37.5 mg/m3) (ACGIH). |
| Dielectric constant | 4.1(2℃) |
| Stability: | Volatile |
| LogP | -0.170 |
| Surface tension | 26.10mN/m at 298.15K |
| CAS DataBase Reference | 64-19-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid(64-19-7) |
| EPA Substance Registry System | Acetic acid (64-19-7) |
Description and Uses
Acetic acid is a colourless liquid or crystal with a sour, vinegar-like odour and is one of the simplest carboxylic acids and is an extensively used chemical reagent. Acetic acid has wide application as a laboratory reagent, in the production of cellulose acetate mainly for photographic film and polyvinyl acetate for wood glue, synthetic fibres, and fabric materials. Acetic acid has also been of large use as a descaling agent and acidity regulator in food industries.
Acetic acid is an important industrial chemical. The reaction of acetic acid with hydroxyl containing compounds, especially alcohols, results in the formation of acetate esters. The largest use of acetic acid is in the production of vinyl acetate . Vinyl acetate can be produced through the reaction of acetylene and acetic acid. It is also produced from ethylene and acetic acid. Vinyl acetate is polymerized into polyvinyl acetate (PVA), which is used in the production of fibers, films, adhesives, and latex paints.
Cellulose acetate, which is used in textiles and photographic film, is produced by reacting cellulose with acetic acid and acetic anhydride in the presence of sulfuric acid. Other esters of acetic acid, such as ethyl acetate and propyl acetate, are used in a variety of applications.
Acetic acid is used to produce the plastic polyethylene terephthalate (PET) . Acetic acid is used to produce pharmaceuticals.
Safety
| Symbol(GHS) |   GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H226-H314 |
| Precautionary statements | P210-P233-P240-P280-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | C,Xi |
| Risk Statements | 34-42-35-10-36/38 |
| Safety Statements | 26-36/37/39-45-23-24/25 |
| RIDADR | UN 1792 8/PG 2 |
| OEB | A |
| OEL | TWA: 10 ppm (25 mg/m3), STEL: 15 ppm (37 mg/m3) |
| WGK Germany | 3 |
| RTECS | NN1650000 |
| F | 1-8-10 |
| Autoignition Temperature | 426 °C |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 29152100 |
| Hazardous Substances Data | 64-19-7(Hazardous Substances Data) |
| Toxicity | LD50 in rats (g/kg): 3.53 orally (Smyth) |
| IDLA | 50 ppm |