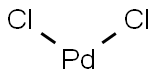Palladium Standard , 1000ug/mlin2.0mol/Lhydrochloricacid , 7647-10-1
Synonym(s):
Palladium dichloride, Hydrochloric acid palladium salt, Palladium Chloride;Palladium(II) chloride (59% Pd)
CAS NO.:7647-10-1
Empirical Formula: Cl2Pd
Molecular Weight: 177.33
MDL number: MFCD00003558
EINECS: 231-596-2
| Pack Size | Price | Stock | Quantity |
| 50ML | RMB734.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 678-680 °C(lit.) |
||||||||||||||
| Density | 4 g/mL at 25 °C(lit.) |
||||||||||||||
| vapor pressure | 0Pa at 20℃ |
||||||||||||||
| storage temp. | Store below +30°C. |
||||||||||||||
| solubility | 55.6g/l insoluble |
||||||||||||||
| form | Powder/Solid |
||||||||||||||
| color | Yellow |
||||||||||||||
| Specific Gravity | 4 |
||||||||||||||
| Odor | Odorless |
||||||||||||||
| PH | 2.15 (30g/l, H2O, 20℃) |
||||||||||||||
| Water Solubility | Insoluble |
||||||||||||||
| Decomposition | 200 °C |
||||||||||||||
| crystal system | Nogata |
||||||||||||||
| Merck | 14,6990 |
||||||||||||||
| Space group | Pnmn |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Stability: | Stable. Incompatible with strong oxidizing agents. |
||||||||||||||
| CAS DataBase Reference | 7647-10-1(CAS DataBase Reference) |
||||||||||||||
| EPA Substance Registry System | Palladium dichloride (7647-10-1) |
Description and Uses
Palladium chloride is a commonly used precious metal catalysts, molecular formula is PaCl2, the appearance is brown-red needle-like crystals or powder, easily deliquescence, the relative density is 4.0 (18 ℃), melting point is 500 ℃ (decomposition), soluble in water, ethanol, acetone and hydrogen bromide. Decomposition in ammonia chloride, potassium iodide, ammonia solution, and precipitation of palladium.
[Uses]
(1)used as the analysis reagents, such as determination of trace palladium, mercury, thallium, iodine, etc.
(2) palladium test strips is used to test carbon monoxide.
(3) also used to search for cracks of buried underground gas pipeline cracks, study of agricultural plant resources, preparation of palladium catalyst, electroplating watch parts and photography, and so on.
[Preparation method] by melting palladium dichloride hydrate, make it lost part of chloride to get Palladium chlorine finished products.
Figure 1 the molecular structure of Palladium chloride.
Palladium chloride is used in photography; toning solutions; electroplating parts of docks and watches; detecting carbon monoxide leaks in buried gas pipes; manufacture of indelible ink; preparation of metal for use as a catalyst; catalyst in jewelry; in dental alloys.
Safety
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H290-H302-H317-H318-H410 |
| Precautionary statements | P234-P273-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | C,Xi,T+,T,Xn |
| Risk Statements | 34-43-40-28-41-37/38-25-22 |
| Safety Statements | 26-36/37/39-45-37/39-28-27-36/37-27/28 |
| RIDADR | UN 1789 8/PG 3 |
| WGK Germany | 2 |
| RTECS | RT3500000 |
| F | 3 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 28439090 |
| Hazardous Substances Data | 7647-10-1(Hazardous Substances Data) |
| Toxicity | MLD i.v. in rabbits: 0.0186 g/kg (Orestano) |