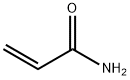
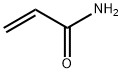
Acrylamide , Super pure level, 99.9% , 79-06-1
Synonym(s):
2-Propenamide;Acrylamide;Acrylic acid amide;Prop-2-enamide
CAS NO.:79-06-1
Empirical Formula: C3H5NO
Molecular Weight: 71.08
MDL number: MFCD00008032
EINECS: 201-173-7
PRODUCT Properties
| Melting point: | 82-86 °C(lit.) |
| Boiling point: | 125 °C25 mm Hg(lit.) |
| bulk density | 500kg/m3 |
| Density | 1,322 g/cm3 |
| vapor density | 2.45 (vs air) |
| vapor pressure | 0.03 mm Hg ( 40 °C) |
| refractive index | 1.460 |
| Flash point: | 138 °C |
| storage temp. | 2-8°C |
| solubility | 2040 g/L (25°C) |
| pka | 15.35±0.50(Predicted) |
| form | powder |
| color | White |
| Odor | Odorless solid |
| PH | 5.0-7.0 (50g/l, H2O, 20℃) |
| Water Solubility | Acrylamide is routinely tested at 250 mg/mL in water, giving a clear colorless solution. It is soluble at least to 40% (w/v) in water, and reportedly up to 215 g/100 mL in water at 30°C. |
| Sensitive | Light Sensitive |
| Merck | 14,129 |
| BRN | 605349 |
| Henry's Law Constant | (x 10-9 atm?m3/mol):
3.03 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: TWA 0.03, IDLH:
60; OSHA PEL: TWA 0.3; ACGIH TLV: TWA 0.03. |
| Stability: | Unstable. Do not heat above 50C. Explosive. Incompatible with acids, bases, oxidizing agents, reducing agents, iron and iron salts, copper, aluminium, brass, free radical initiators. Air sensitive. Hygroscopic. |
| InChIKey | HRPVXLWXLXDGHG-UHFFFAOYSA-N |
| LogP | -0.9 at 20℃ and pH7 |
| CAS DataBase Reference | 79-06-1(CAS DataBase Reference) |
| IARC | 2A (Vol. 60, Sup 7) 1994 |
| NIST Chemistry Reference | Acrylamide(79-06-1) |
| EPA Substance Registry System | Acrylamide (79-06-1) |
Description and Uses
Acrylamide is an odorless, white crystalline solid that initially
was produced for commercial purposes by reaction of acrylonitrile
with hydrated sulfuric acid.
Acrylamide exists in two forms: a monomer and a polymer.
Monomer acrylamide readily participates in radicalinitiated
polymerization reactions, whose products form the
basis of most of its industrial applications. The single unit
form of acrylamide is toxic to the nervous system, a carcinogen
in laboratory animals and a suspected carcinogen in
humans. The multiple unit or polymeric form is not known to
be toxic.
Acrylamide is formed as a by-product of the Maillard reaction.
The Maillard reaction is best known as a reaction that
produces pleasant flavor, taste, and golden color in fried and
baked foods; the reaction occurs between amines and carbonyl
compounds, particularly reducing sugars and the amino acid
asparagine. In the first step of the reaction, asparagine reacts
with a reducing sugar, forming a Schiff’s base. From this
compound, acrylamide is formed following a complex reaction
pathway that includes decarboxylation and a multistage elimination
reaction. Acrylamide formation in bakery products,
investigated in a model system, showed that free asparagine
was a limiting factor. Treatment of flours with asparaginase
practically prevented acrylamide formation. Coffee drinking
and smoking are other major sources apart from the human
diet.
In the production of polyacrylamides, which are used in water and waste treatment, paper and pulp processing, cosmetic additives, and textile processing; in adhesives and grouts; as cross-linking agents in vinyl polymers
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301-H312+H332-H315-H317-H319-H340-H350-H361f-H372 |
| Precautionary statements | P202-P280-P301+P310-P302+P352+P312-P304+P340+P312-P305+P351+P338 |
| Hazard Codes | T |
| Risk Statements | 45-46-20/21-25-36/38-43-48/23/24/25-62-48/20/21/22-22-24/25 |
| Safety Statements | 53-45-24-36/37/39-26-36/37 |
| OEB | D |
| OEL | TWA: 0.03 mg/m3 [skin] |
| RIDADR | UN 3426 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | AS3325000 |
| F | 8-10 |
| Autoignition Temperature | 424°C |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29241900 |
| Hazardous Substances Data | 79-06-1(Hazardous Substances Data) |
| Toxicity | LD50 i.p. in mice: 170 mg/kg (Peterson, Sheth) |
| IDLA | 60 mg/m3 |