Ttinchloride , 1.0Minmethylenechloride , 7646-78-8
Synonym(s):
Stannic chloride;Stannic chloride fuming;Tin tetrachloride
CAS NO.:7646-78-8
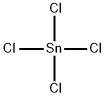
Empirical Formula: Cl4Sn
Molecular Weight: 260.52
MDL number: MFCD00011242
EINECS: 231-588-9
PRODUCT Properties
| Melting point: | -33 °C (lit.) |
| Boiling point: | 114 °C (lit.) |
| Density | 2.226 g/mL at 25 °C (lit.) |
| vapor density | 9 (vs air) |
| vapor pressure | 10 mm Hg ( 10 °C) |
| refractive index | 1.512 |
| Flash point: | 34 °F |
| storage temp. | Store at RT. |
| solubility | Miscible with alcohol, benzene, toluene, chloroform, acetone, carbon terachloride, gasoline and carbon disulfide. |
| form | Solution |
| Specific Gravity | 2.226 |
| color | Colorless |
| PH | 0.2 (60g/l, H2O, 20℃)Hydrolysis |
| Water Solubility | reacts |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Sensitive | Air Sensitive |
| Merck | 14,8774 |
| Exposure limits | ACGIH: TWA 50 ppm OSHA: TWA 25 ppm; STEL 125 ppm NIOSH: IDLH 2300 ppm |
| Dielectric constant | 3.2(22℃) |
| Stability: | Stability Stable, but may decompose upon exposure to moist air or water. Incompatible with strong bases, alcohols. |
| LogP | -1.67 at 20℃ |
| CAS DataBase Reference | 7646-78-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Tin tetrachloride(7646-78-8) |
| EPA Substance Registry System | Stannane, tetrachloro- (7646-78-8) |
Description and Uses
Tin (IV) chloride appears as white crystals with a strong pungent chlorine odour. On heating, tin (IV) chloride decomposition emits acrid fumes. At room temperature, it is colourless and releases fumes on contact with air, giving a stinging odour. Stannic chloride was used as a chemical weapon during World War I. It is also used in the glass container industry for making an external coating that toughens the glass. Stannic chloride is used in chemical reactions with fuming (90%) nitric acid for the selective nitration of activated aromatic rings in the presence of unactivated ones. Tin (IV) chloride reacts violently with water or moist air to produce corrosive hydrogen chloride. Tin (IV) chloride reacts with turpentine, alcohols, and amines, causing fire and explosion hazard. It attacks many metals, some forms of plastic, rubber, and coatings.
Tin(IV) chloride is a precursor to prepare organotin compounds such as tetralkyltin and dialkyldichlorotin(IV), which find applications as catalysts and polymer stabilizers. As a Lewis acid catalyst, it is used in Fridel-Crafts reactions for alkylation and cyclization. It is involved in the selective nitration of aromatic compounds in the presence of fuming nitric acid. Furthermore, it is used to prepare tin(IV) oxide coating by sol-gel process.
Safety
| Symbol(GHS) |   GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H314-H335-H412 |
| Precautionary statements | P261-P271-P273-P280-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | C,T,F,N |
| Risk Statements | 34-52/53-40-23/24/25-11-67-37-65-50/53 |
| Safety Statements | 26-45-61-7/8-36/37-24/25-23-36/37/39-16-62 |
| RIDADR | UN 3264 8/PG 2 |
| WGK Germany | 2 |
| RTECS | XP8750000 |
| F | 10-21 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28273990 |
| Hazardous Substances Data | 7646-78-8(Hazardous Substances Data) |